Fresh from the podium at the ESMO 2025 Congress, the RIBOLARIS trial was presented by Dr. Paul H. Cottu (Paris, France), unveiling new data on the use of ribociclib plus endocrine therapy (ET) in early hormone receptor–positive, HER2-negative (HR+/HER2−) breast cancer. This multicenter study evaluated whether patients achieving a low Risk of Recurrence (ROR) score—derived from the PAM50 genomic assay—after neoadjuvant ribociclib plus ET could safely omit adjuvant chemotherapy. The trial builds on previous research from NeoPAL and CORALLEEN, which explored the role of CDK4/6 inhibitors combined with ET as an alternative to chemotherapy in the neoadjuvant setting.
Background
CDK4/6 inhibitors have become a cornerstone in the management of hormone receptor–positive, HER2-negative (HR+/HER2−) early breast cancer, providing an effective and well-tolerated alternative to traditional chemotherapy. Randomized clinical trials such as NeoPAL and CORALLEEN have shown that combining CDK4/6 inhibitors with endocrine therapy achieves comparable biological and clinical activity to multi-agent chemotherapy, particularly in patients with luminal B (PAM50) subtype disease. These findings have shifted the therapeutic landscape, highlighting the potential of targeted and endocrine-based strategies in reducing treatment toxicity without compromising efficacy.
The PAM50-based Risk of Recurrence (ROR) score has emerged as a powerful biomarker to assess residual disease biology and predict prognosis after neoadjuvant CDK4/6i–ET, providing a molecular tool to guide adjuvant treatment decisions. In this context, the RIBOLARIS trial was designed to evaluate whether patients who achieve a low ROR score following neoadjuvant ribociclib combined with endocrine therapy could safely omit adjuvant chemotherapy, aiming to personalize treatment intensity based on genomic response and minimize unnecessary exposure to cytotoxic agents.
Methods
RIBOLARIS is an open-label, single-arm, multicenter trial enrolling patients with operable stage II, grade 2/3, Ki67 ≥20% HR+/HER2− BC eligible for adjuvant chemotherapy. Participants received six cycles of neoadjuvant RIB-ET (ribociclib 600 mg/day; 3 weeks on/1 week off + letrozole 2.5 mg/day), followed by surgery within 10 days. The PAM50 ROR score guided adjuvant management: patients with ROR-low tumors continued RIB-ET, while those with ROR-medium/high tumors received chemotherapy-based treatment followed by RIB-ET. The prespecified interim analysis assessed safety and efficacy after 686 surgeries, with ≥40% of patients expected to reach a ROR-low score.
Results
At data cut-off, 686 of 1100 planned surgeries (62.4%) were completed. Baseline characteristics: median age 57 years (range 38–84), postmenopausal 62%, stage IIA 60%, node-negative 60%, and histologic grade 2 in 74%.
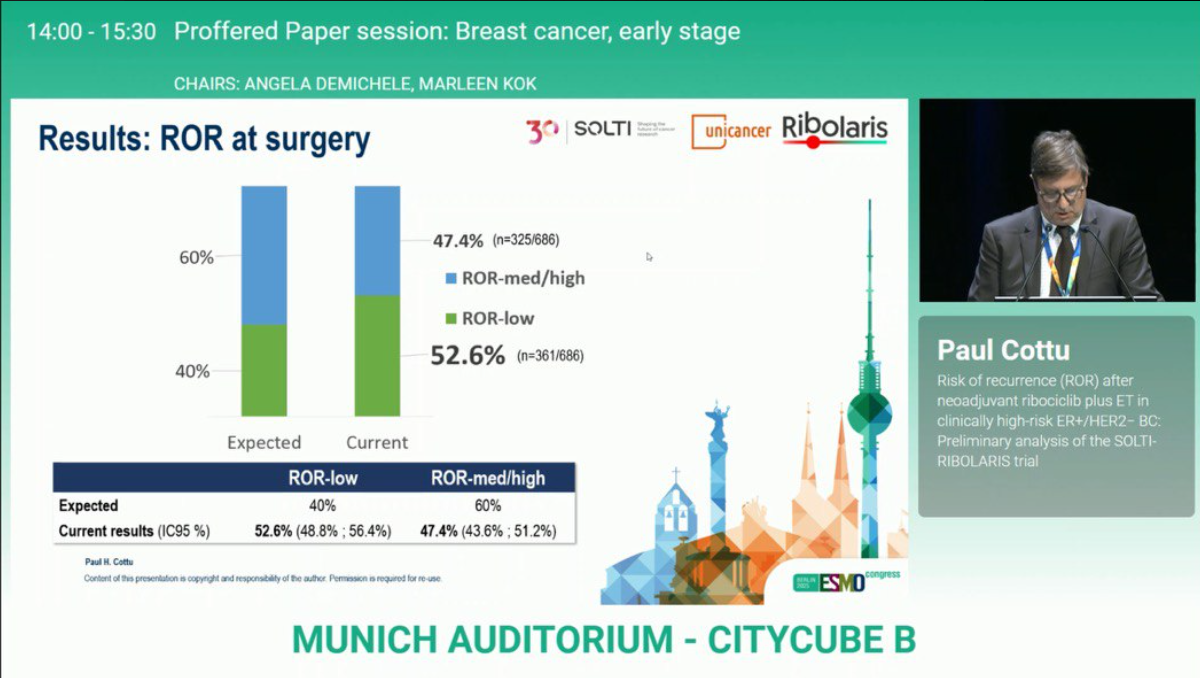
- Following six cycles of neoadjuvant ribociclib plus endocrine therapy (RIB-ET), 52.6% of patients (n=361) achieved a low Risk of Recurrence (ROR-low) score, with a mean of 11.3 (95% CI 10.5–12.2).
- The remaining 47.4% (n=325) demonstrated medium or high ROR scores, with a mean of 36.9 (95% CI 34.2–39.5), indicating a nearly even distribution between low and higher molecular risk groups.
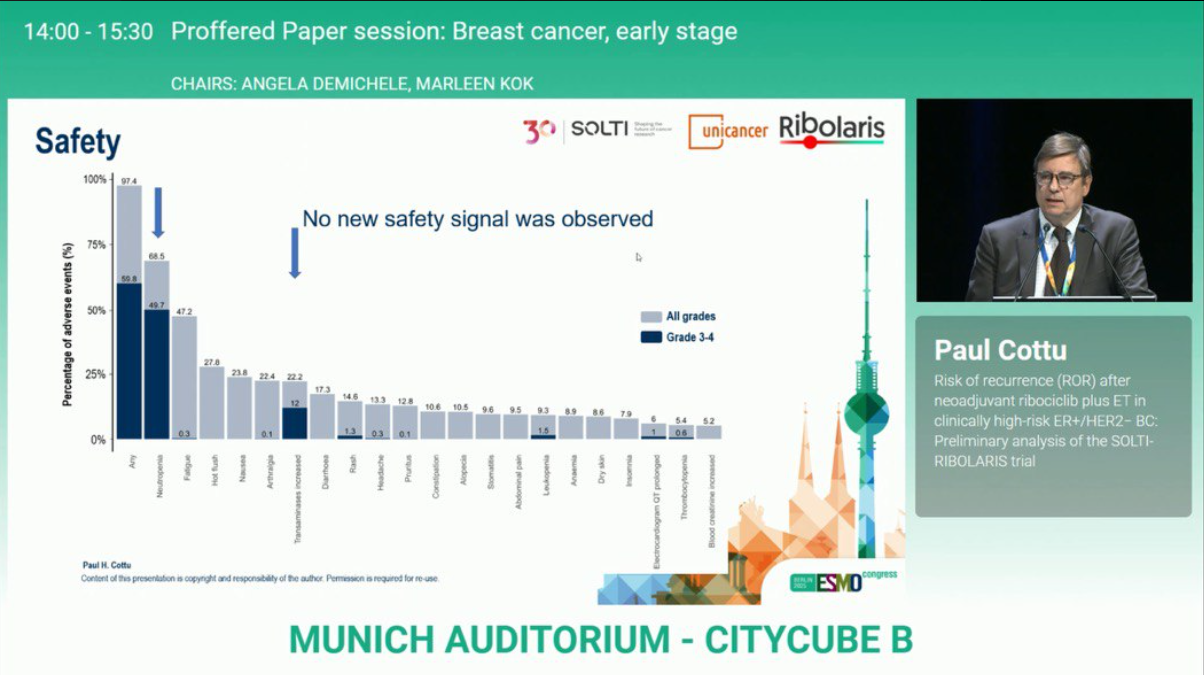
- The most frequent grade 3–4 treatment-emergent adverse events (TEAEs) were neutropenia (grade 3: 46.3%; grade 4: 3.5%) and elevated transaminases (grade 3: 10.4%; grade 4: 1.5%). No unexpected toxicities or new safety signals were observed.
Conclusions
The interim analysis of the RIBOLARIS trial confirms and extends the findings of earlier studies such as CORALLEEN and NeoPAL, reinforcing the potential role of CDK4/6 inhibition combined with endocrine therapy as an effective alternative to chemotherapy in selected patients with early HR+/HER2− breast cancer. The results demonstrated that a substantial proportion of patients achieved a low Risk of Recurrence (ROR-low) score after neoadjuvant treatment with ribociclib and endocrine therapy, indicating a favorable molecular response.
These findings support a more tailored treatment approach, suggesting that patients who reach a ROR-low status may be safely spared adjuvant chemotherapy without compromising efficacy. Importantly, no new or unexpected safety signals were observed, confirming the manageable toxicity profile of ribociclib-based therapy in the neoadjuvant setting.

You can read the full abstract here.


