The term “Red Devil Chemotherapy” has long served as a vivid nickname in oncology circles, referring primarily to doxorubicin, a deep red anthracycline chemotherapy agent known for its remarkable efficacy and equally formidable toxicity profile. The bright hue of the drug, visible even in intravenous preparations, and its association with significant side effects—most notably cardiotoxicity—have contributed to its ominous reputation. Over time, the phrase “Red Devil” has come to symbolize the critical balance clinicians must strike between therapeutic potency and the potential for lasting harm.
Although doxorubicin is the most frequently linked agent with this moniker, it is not alone in its class. Other structurally related, red-colored anthracyclines—such as epirubicin, idarubicin, and daunorubicin—are also considered part of this category. These agents play a foundational role in the treatment of breast cancer, lymphomas, leukemias, and soft tissue sarcomas, among other malignancies. Their shared mechanism of action and toxicity profiles, especially regarding cumulative cardiac damage, make them both life-saving and potentially life-threatening tools in the oncologist’s arsenal.
This article will take a closer look at how “Red Devil” chemotherapy drugs like doxorubicin and its related agents (epirubicin, idarubicin, and daunorubicin) work, what types of cancer they treat, and why they are both powerful and risky. It will explain their effects on the body, especially the heart, and describe how doctors try to reduce side effects while still treating the cancer effectively. The article will also highlight new developments—like liposomal versions and smarter combinations—that aim to make these treatments safer and more targeted for each patient.
Structural Features and Pharmacologic Advances of Anthracycline Chemotherapy
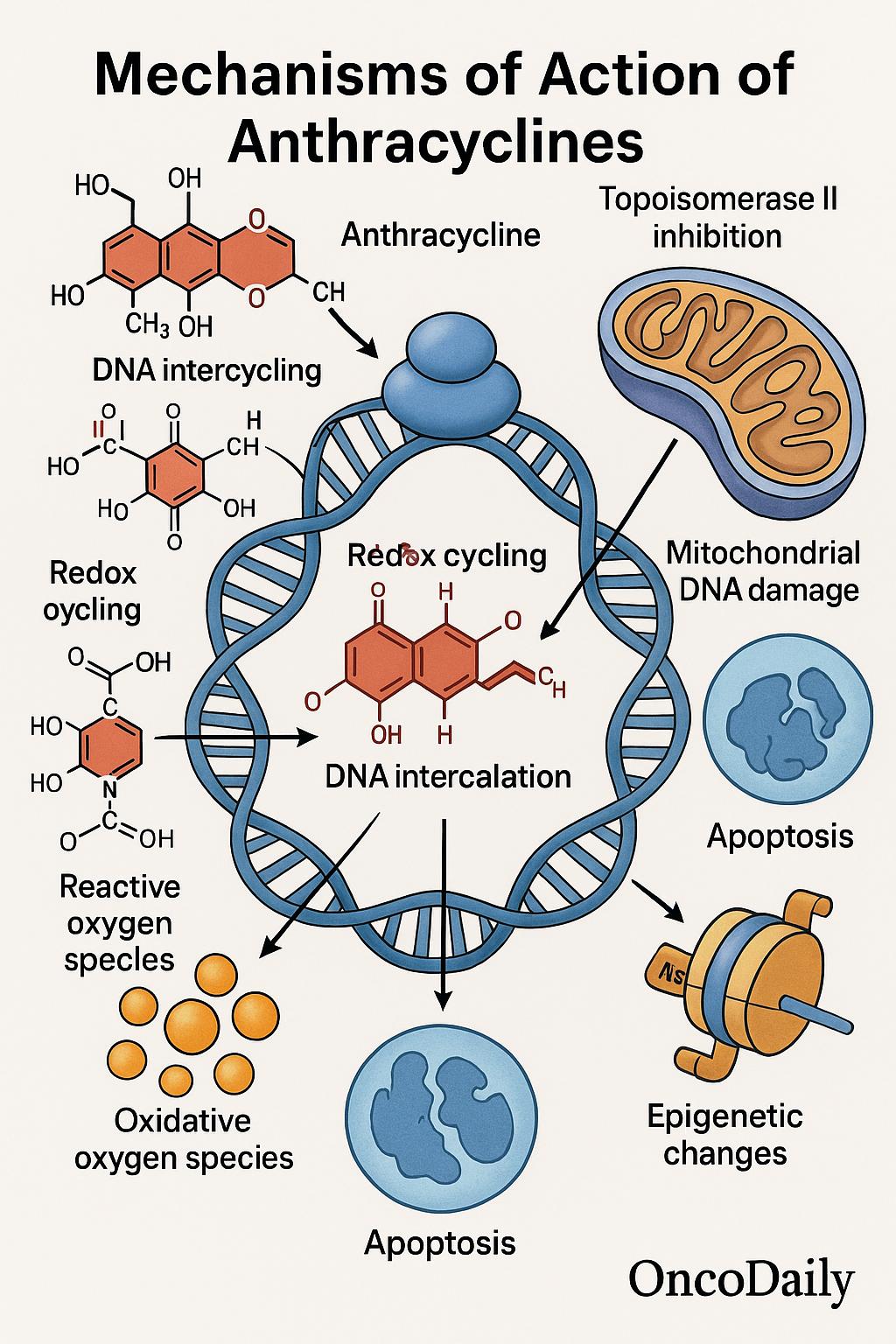
Anthracyclines are a class of chemotherapeutic agents characterized by a shared molecular backbone consisting of a tetracyclic ring structure—specifically, an anthraquinone core linked to a sugar moiety, usually daunosamine. Central to their cytotoxic mechanism is the quinone/hydroquinone moiety within this tetracyclic system, which undergoes redox cycling and generates reactive oxygen species (ROS). These free radicals inflict oxidative damage on cellular components, including DNA and lipid membranes, contributing significantly to the antitumor activity of these agents—though also implicated in dose-dependent cardiotoxicity.
The development of liposomal formulations, such as pegylated liposomal doxorubicin (PLD), represents a major advancement in anthracycline pharmacology. By encapsulating the drug in liposomes and coating them with polyethylene glycol (PEG), PLD alters the pharmacokinetics of doxorubicin. It achieves prolonged circulation time, reduced peak plasma concentrations, and preferential tumor accumulation via the enhanced permeability and retention (EPR) effect. Importantly, this formulation significantly lowers the risk of cardiotoxicity and mucosal toxicity, making it a preferred choice in patients with comorbidities or prior exposure to anthracyclines.
Differences among anthracyclines influence their clinical applications. For example, idarubicin is more lipophilic than doxorubicin or daunorubicin, allowing improved cellular uptake and better central nervous system (CNS) penetration—an advantage in treating leukemias with CNS involvement. Epirubicin, structurally similar to doxorubicin but with an epimerized hydroxyl group, demonstrates reduced cardiotoxicity while retaining efficacy in breast and gastric cancers. Understanding these structural nuances and formulation strategies is critical in optimizing anthracycline use while minimizing associated toxicities.
Mechanism of Action of Anthracyclines: DNA Damage, Oxidative Stress, and Beyond
Anthracyclines exert their potent anticancer activity through multiple, interrelated mechanisms that target the integrity and function of DNA. The central and most well-established mechanism involves inhibition of topoisomerase II, an essential nuclear enzyme that relieves torsional stress during DNA replication. By stabilizing the transient DNA-topoisomerase II cleavage complex, anthracyclines prevent re-ligation of double-stranded DNA breaks, ultimately leading to apoptosis.
In addition to this, anthracyclines such as doxorubicin and daunorubicin engage in DNA intercalation, slipping between base pairs of the DNA helix. This action disrupts essential processes such as transcription and replication, further amplifying genotoxic stress within rapidly dividing tumor cells.
A defining and unique feature of anthracyclines is their ability to generate reactive oxygen species (ROS) through redox cycling of the quinone moiety. This oxidative stress contributes to both their antitumor activity and their notorious dose-limiting cardiotoxicity, as cardiac myocytes are especially susceptible to ROS due to relatively low levels of endogenous antioxidant enzymes like catalase.
Emerging research has also illuminated previously underappreciated mechanisms, including damage to mitochondrial DNA, which compromises cellular respiration and can activate intrinsic apoptotic pathways. Moreover, anthracyclines have been shown to induce epigenetic modifications, such as changes in histone acetylation and methylation, which can influence gene expression and chromatin organization in ways that may contribute to both therapeutic efficacy and long-term cellular dysregulation.
Clinical Applications of Red Chemotherapy Agents in Solid and Hematologic Malignancies
Anthracyclines, including doxorubicin, epirubicin, daunorubicin, and idarubicin, remain foundational agents across a wide spectrum of solid and hematologic malignancies. In breast cancer, doxorubicin is frequently used in early-stage and locally advanced settings, often as part of the dose-dense AC (doxorubicin and cyclophosphamide) followed by taxane (T) regimen, which remains a preferred NCCN-recommended protocol for HER2-negative disease. For triple-negative breast cancer, this sequence is often combined with immune checkpoint blockade in neoadjuvant settings per NCCN Guidelines Version 1.2025.
In aggressive lymphomas such as diffuse large B-cell lymphoma (DLBCL), doxorubicin is a core component of the R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), which remains the frontline standard per NCCN Guidelines. The omission of doxorubicin due to cardiotoxicity concerns is generally avoided unless clinically necessary, as its inclusion has been shown to significantly impact survival.
In hematologic malignancies, particularly acute myeloid leukemia (AML), daunorubicin in combination with cytarabine constitutes the traditional “7+3” induction regimen. For high-risk or relapsed AML cases, intensified regimens or liposomal formulations like CPX-351 (liposomal daunorubicin + cytarabine) may be used to enhance delivery while modulating toxicity.
Soft tissue sarcomas also frequently rely on anthracycline therapy. Doxorubicin monotherapy remains the first-line treatment for advanced or unresectable soft tissue sarcomas according to NCCN Guidelines Version 2.2025. In some subtypes or cases requiring aggressive control, doxorubicin may be combined with ifosfamide, though at the cost of increased hematologic and renal toxicity.
Toxicity Profile of Red Chemotherapy: Cardiotoxicity and Systemic Complications
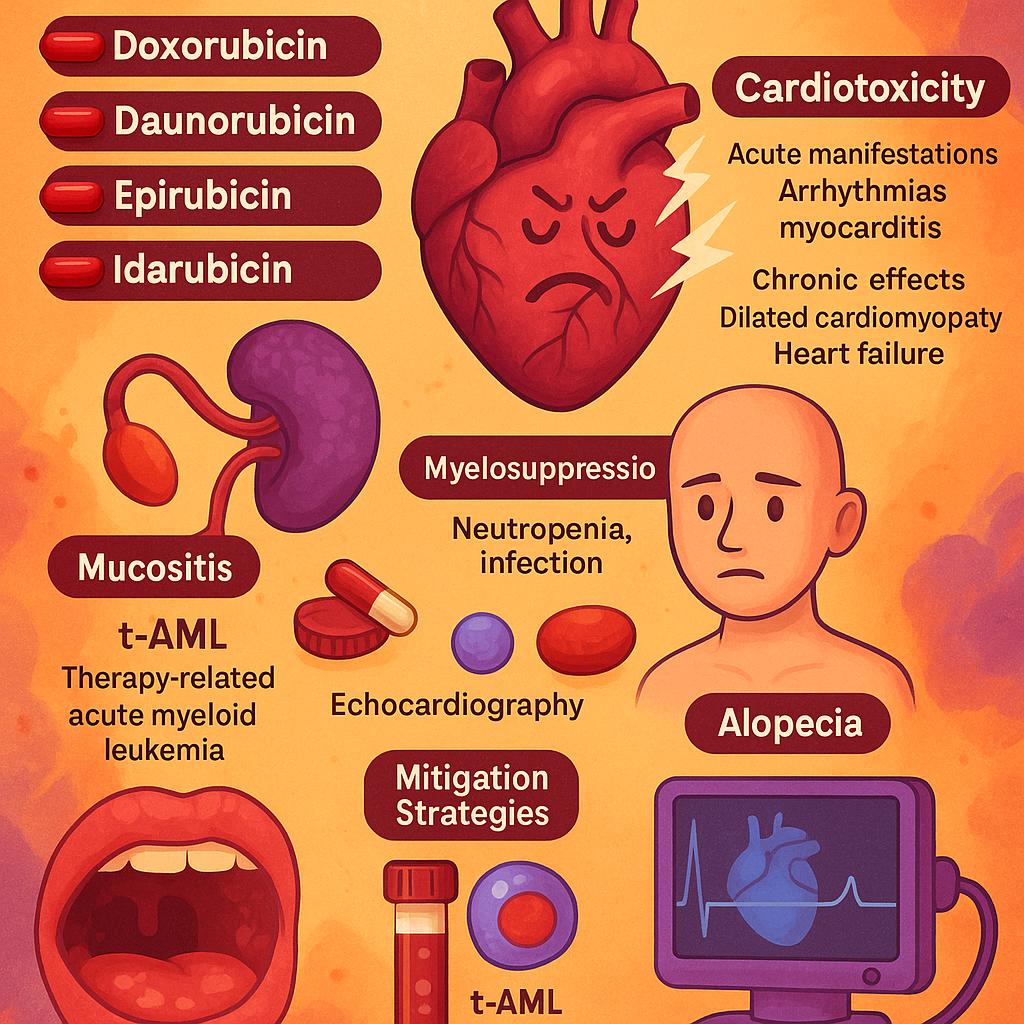
Anthracyclines such as doxorubicin, daunorubicin, epirubicin, and idarubicin are potent agents in oncology but are also associated with a well-characterized and significant toxicity profile. Among these, cardiotoxicity stands out as the most clinically limiting adverse effect, often determining the upper threshold of cumulative dosing. Acute manifestations can include arrhythmias and myocarditis, typically occurring within hours to days after administration. However, it is the chronic cardiotoxicity—manifesting as dilated cardiomyopathy and congestive heart failure—that poses the greatest long-term concern, especially when cumulative doxorubicin doses exceed 450–550 mg/m².
In addition to cardiac toxicity, myelosuppression is a universal and dose-limiting toxicity of anthracyclines, leading to neutropenia and increased infection risk. Mucositis and alopecia are also commonly observed and significantly affect patients’ quality of life. A particularly concerning late complication is therapy-related acute myeloid leukemia (t-AML), which has been notably associated with agents such as idarubicin and daunorubicin, especially when used in combination with alkylating agents or topoisomerase II inhibitors.
Several strategies have been developed to mitigate these toxicities. Dexrazoxane, an iron-chelating agent, is FDA-approved for cardioprotection in patients receiving high cumulative doses of doxorubicin and has shown efficacy in reducing cardiac events without significantly compromising antitumor activity. Liposomal formulations, such as pegylated liposomal doxorubicin, alter drug pharmacokinetics to reduce cardiac uptake and therefore diminish the risk of cardiotoxicity. Regular cardiac monitoring using echocardiography—including strain imaging and global longitudinal strain assessment—has become standard for patients at risk, allowing for early detection of subclinical dysfunction.
Mechanisms of Resistance to Red Chemotherapy
Despite the potent cytotoxicity of anthracyclines, many tumors eventually develop mechanisms of resistance that compromise treatment efficacy. One of the most well-established pathways involves the overexpression of ATP-binding cassette (ABC) transporters, particularly P-glycoprotein (P-gp), encoded by the MDR1 gene. This efflux pump actively transports doxorubicin and related drugs out of cancer cells, reducing intracellular concentrations to subtherapeutic levels.
Another key mechanism is the downregulation or mutation of topoisomerase IIα—the primary target of anthracyclines. When expression of this enzyme is reduced or its function is altered, the ability of anthracyclines to induce double-stranded DNA breaks is significantly impaired, thereby blunting their cytotoxic effects. Detoxification pathways also contribute to resistance. Elevated activity of glutathione S-transferase (GST) and related enzymes enhances the cellular capacity to neutralize reactive oxygen species and drug metabolites, limiting oxidative damage induced by anthracyclines.
Furthermore, the tumor microenvironment itself can foster resistance. Hypoxic conditions within poorly vascularized tumor regions reduce drug delivery and limit the generation of reactive oxygen species, which require oxygen for maximal damage. Stromal components, such as cancer-associated fibroblasts and extracellular matrix proteins, can create physical and biochemical barriers to drug penetration, while also secreting survival factors that shield tumor cells from chemotherapy-induced apoptosis.
Overcoming the Limitations of Anthracyclines: Advances in Formulations and Combinations
To mitigate the well-documented toxicities and resistance mechanisms associated with anthracyclines, particularly doxorubicin, researchers have developed innovative approaches aimed at enhancing therapeutic efficacy while reducing harm to healthy tissues.
One of the most clinically successful advances has been the development of liposomal formulations, such as pegylated liposomal doxorubicin (PLD). These formulations encapsulate the drug in a liposome with a polyethylene glycol (PEG) coating, altering its pharmacokinetics to favor tumor accumulation via the enhanced permeability and retention (EPR) effect. In the CALYPSO trial, PLD combined with carboplatin showed superior progression-free survival and a better safety profile—especially reduced alopecia and neutropenia—compared to conventional paclitaxel-carboplatin in recurrent ovarian cancer. Similarly, in metastatic breast cancer, PLD has demonstrated comparable efficacy to conventional doxorubicin with a significantly lower incidence of cardiotoxicity, particularly in elderly or anthracycline-pretreated patients.
In parallel, the field has moved toward targeted delivery of anthracyclines using antibody-drug conjugates (ADCs). These platforms link anthracyclines to monoclonal antibodies against tumor-specific antigens, such as HER2, allowing precise delivery of the cytotoxic payload. Early-phase trials of HER2-targeted anthracycline conjugates show promise, particularly in HER2-low expressing breast cancers, expanding treatment options beyond traditional HER2-positive disease.
Another rapidly evolving area is the combination of anthracyclines with immune checkpoint inhibitors. Preclinical models suggest that anthracyclines can induce immunogenic cell death (ICD), enhancing dendritic cell maturation and T-cell priming. Trials are now underway to assess whether combining doxorubicin with PD-1/PD-L1 inhibitors can synergize to overcome immune resistance, particularly in triple-negative breast cancer (TNBC) and soft tissue sarcomas. Finally, novel anthracycline analogs are being developed and regionally approved. Pirarubicin, a red-colored agent approved in parts of Asia, has demonstrated a more favorable cardiac safety profile while retaining potent antitumor activity, especially in hepatocellular carcinoma and bladder cancer. Other next-generation compounds are in preclinical pipelines, designed to reduce redox cycling or evade efflux transporters, thus improving both safety and efficacy.
Toward Personalized Use of Anthracyclines: Predictive Tools and Biomarkers
Personalizing anthracycline-based chemotherapy has become a critical focus in oncology, aiming to maximize efficacy while minimizing toxicity. Several clinical, genetic, and tumor-specific tools are now being integrated to guide patient selection and dosing strategies.
One of the most established tools in clinical practice is the assessment of baseline cardiac function, especially left ventricular ejection fraction (LVEF) measured by echocardiography. Patients with reduced LVEF at baseline are at significantly higher risk for anthracycline-induced cardiotoxicity and may be candidates for dose adjustment, alternative therapies, or cardioprotective agents like dexrazoxane. Additionally, cardiac biomarkers such as troponin I or T and N-terminal pro–B-type natriuretic peptide (NT-proBNP) can help identify subclinical myocardial injury. Elevated baseline or rising levels during treatment have been associated with increased risk for long-term cardiac dysfunction.
On the genomic front, pharmacogenomic markers are gaining relevance. Polymorphisms in genes encoding drug-metabolizing enzymes have been linked to differential susceptibility to anthracycline toxicity. One notable example is the CBR3 V244M variant—a single nucleotide polymorphism in the carbonyl reductase 3 gene—which has been associated with increased conversion of doxorubicin to its cardiotoxic metabolite, doxorubicinol. Carriers of this allele, particularly in pediatric acute lymphoblastic leukemia cohorts, show higher rates of cardiotoxicity, suggesting that genotyping may inform risk stratification in susceptible populations.
Tumor biology also plays a role in anthracycline responsiveness. For example, in breast cancer, TOP2A gene amplification has emerged as a predictive biomarker of anthracycline benefit. TOP2A encodes topoisomerase IIα, the direct target of doxorubicin, and its amplification has been associated with improved response rates to anthracycline-based regimens. Consequently, molecular profiling of tumors for TOP2A, especially when HER2 amplification is present, may help oncologists determine when the inclusion of an anthracycline adds meaningful clinical value.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD


