PSMAddition (NCT04720157), a phase 3 clinical trial presented by Dr. Scott Tagawa at the ESMO Congress 2025, demonstrated that adding ¹⁷⁷Lu-PSMA-617 to standard androgen deprivation therapy (ADT) and an androgen receptor pathway inhibitor (ARPI) significantly improved radiographic progression-free survival (rPFS) in patients with PSMA-positive metastatic hormone-sensitive prostate cancer (mHSPC).
Background
¹⁷⁷Lu-PSMA-617 is a PSMA-targeted radioligand therapy that binds to prostate-specific membrane antigen on tumor cells and delivers β-particle radiation to destroy them. It has already shown strong benefit in advanced disease through the VISION and PSMAfore studies.PSMAddition is the first phase 3 trial to test this approach earlier, in hormone-sensitive metastatic disease, aiming to delay progression and improve long-term survival outcomes.
Study Design
The trial enrolled 1,144 men with newly diagnosed or minimally treated PSMA-positive mHSPC confirmed by ⁶⁸Ga-PSMA-11 PET/CT. Participants were randomized 1:1 to receive either ¹⁷⁷Lu-PSMA-617 (7.4 GBq every six weeks for six cycles) + ADT + ARPI or ADT + ARPI alone.Patients in the control arm could cross over to ¹⁷⁷Lu-PSMA-617 upon centrally confirmed radiographic progression.
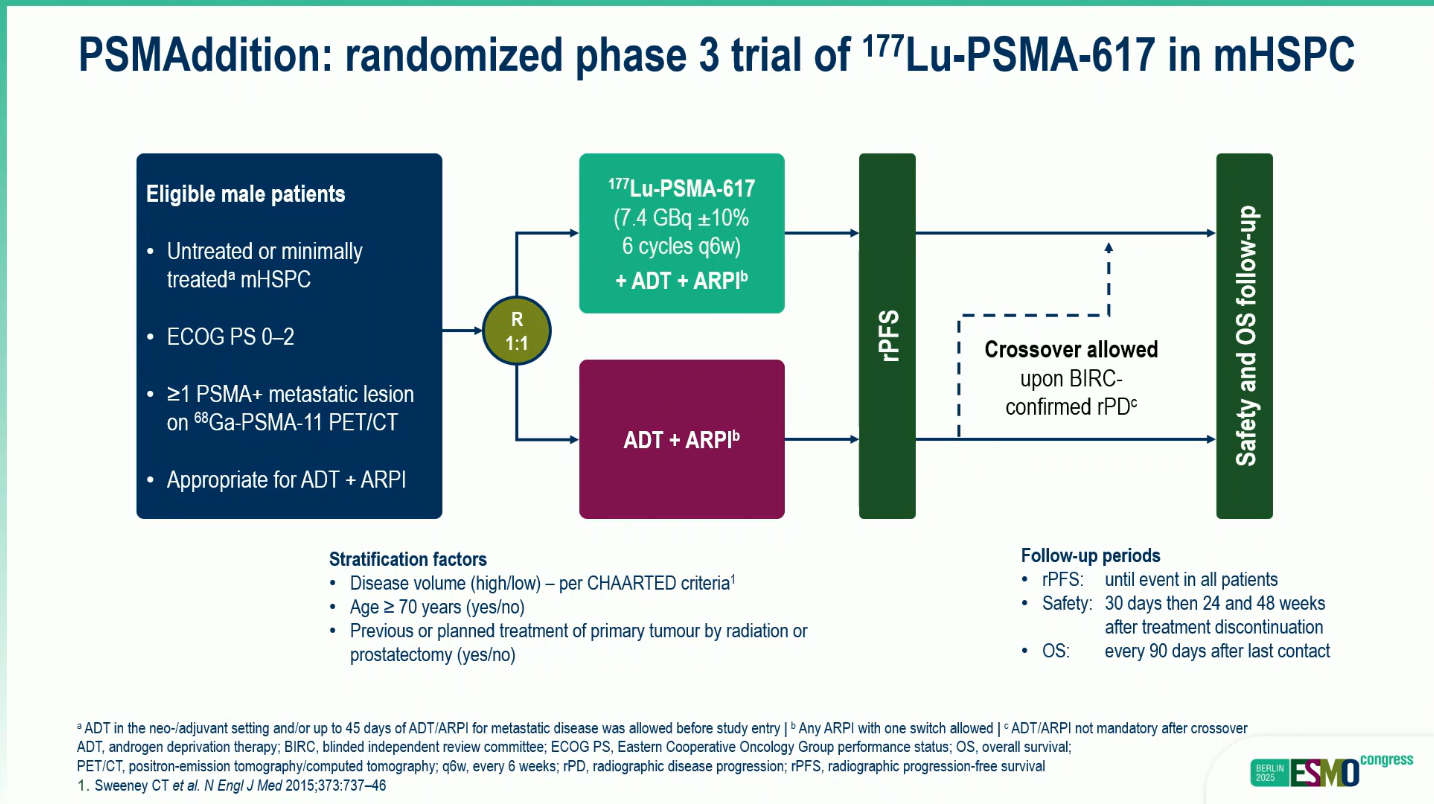
The primary endpoint was rPFS per blinded independent review; secondary endpoints included overall survival (OS), objective response rate (ORR), safety, and quality of life (QoL).
Results
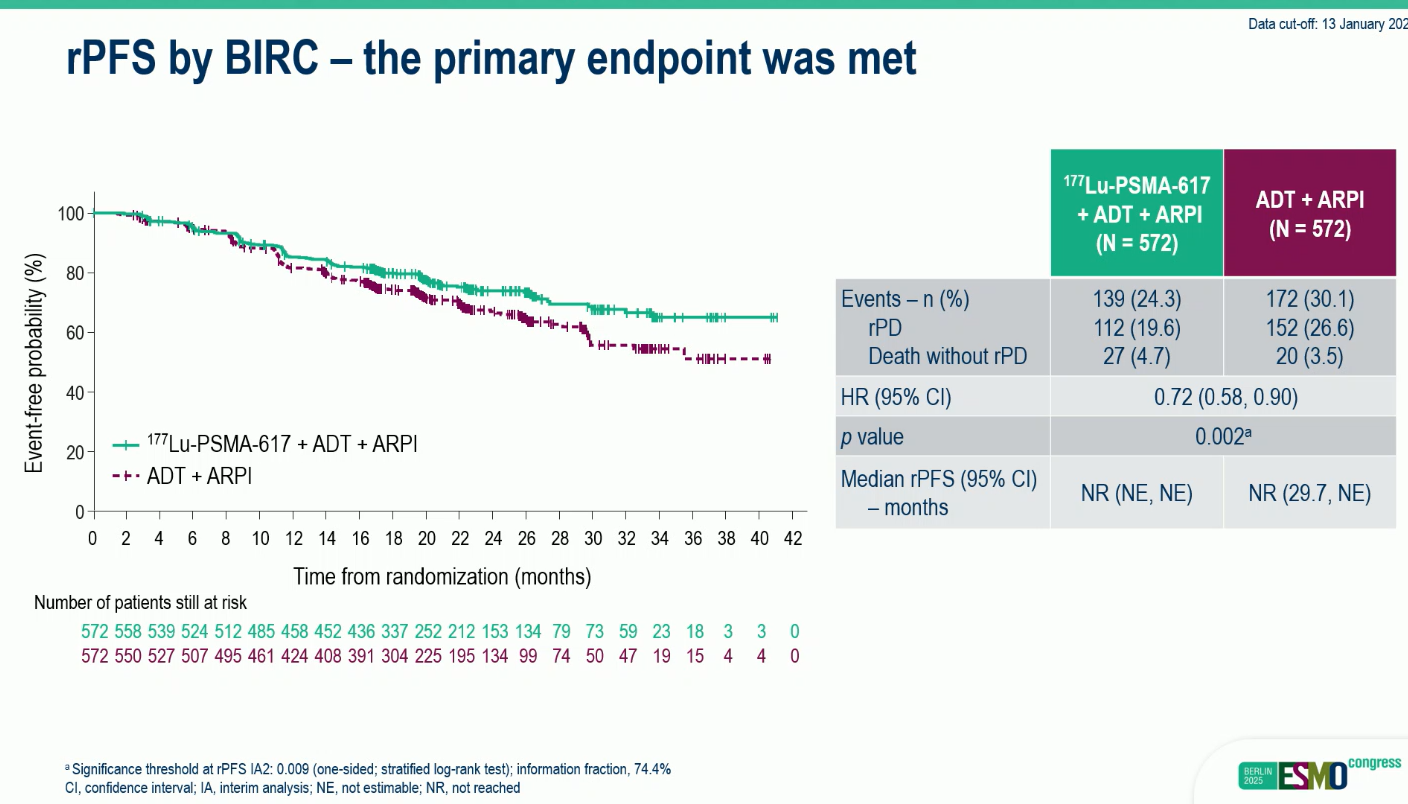
At the time of the second interim analysis (median follow-up 23.6 months), median rPFS and OS were not yet reached in either arm. However, the addition of ¹⁷⁷Lu-PSMA-617 significantly reduced the risk of radiographic progression or death by 28% (HR 0.72; p=0.002), with consistent benefits across key endpoints.
- rPFS: HR 0.72 (95% CI 0.58–0.90; p=0.002).
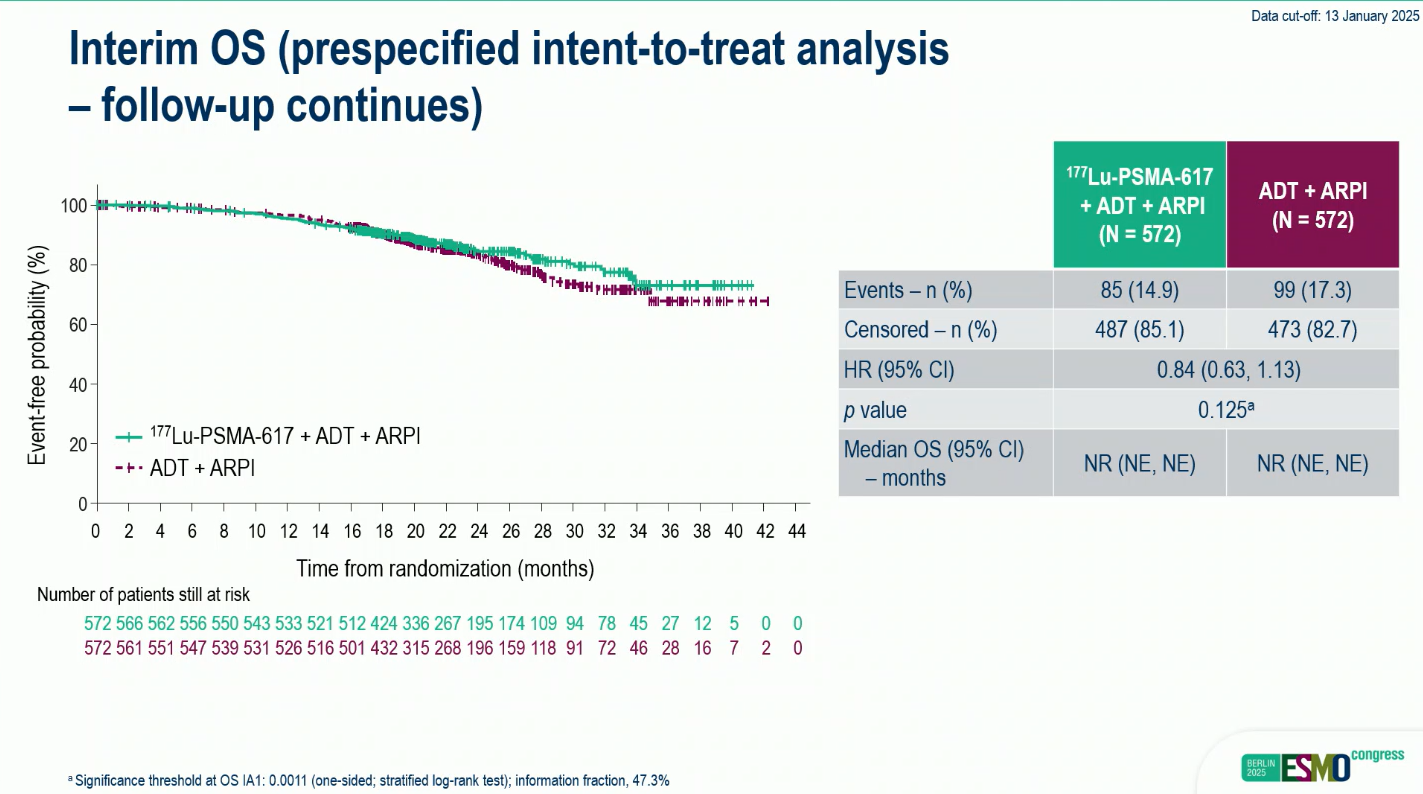
- OS: HR 0.84 (95% CI 0.63–1.13; p=0.125); follow-up ongoing.
- ORR: 85.3% vs 80.8%; complete response 57% vs 42%.
- PSA <0.2 ng/mL at 48 weeks: 87% vs 75%.
- Time to mCRPC: HR 0.70 (95% CI 0.58–0.84).
- Time to PSA progression: HR 0.42 (95% CI 0.30–0.59).
- QoL: No meaningful difference in FACT-P or EQ-5D between arms.
Safety
¹⁷⁷Lu-PSMA-617 was well tolerated and consistent with previous trials.
- Any-grade AEs: 98.4% vs 96.6%.
- Grade ≥3 AEs: 50.7% vs 43.0%, mainly cytopenias (14%).
- Dry mouth: 41% grade 1 and 5% grade 2 vs 3.8% total in control.
- No treatment-related deaths; overall QoL maintained
Conclusion
The PSMAddition trial represents a pivotal advancement in the management of PSMA-positive metastatic hormone-sensitive prostate cancer (mHSPC). By integrating ¹⁷⁷Lu-PSMA-617—a targeted radioligand therapy—into the frontline setting alongside androgen deprivation therapy (ADT) and an androgen receptor pathway inhibitor (ARPI), the study achieved a significant delay in disease progression without compromising safety or quality of life.
These results highlight the potential of early radioligand therapy to fundamentally reshape the treatment paradigm for advanced prostate cancer. Historically, radioligand therapies have been reserved for the castration-resistant stage, when disease burden is higher and responses are less durable. PSMAddition shifts this approach upstream, demonstrating that earlier PSMA-directed treatment can enhance depth and durability of response, delay progression to castration resistance, and potentially improve long-term survival outcomes.
You can read the full abstract here.


