The introduction of immune checkpoint inhibitors (ICIs) has fundamentally altered response kinetics in oncology, creating a mismatch between conventional anatomic imaging and underlying tumor biology. Unlike cytotoxic chemotherapy or targeted therapy—where radiologic tumor shrinkage in responders and progressive growth in non-responders typically occur within predictable timeframes—ICIs can produce atypical response patterns that complicate early treatment assessment and clinical decision-making.
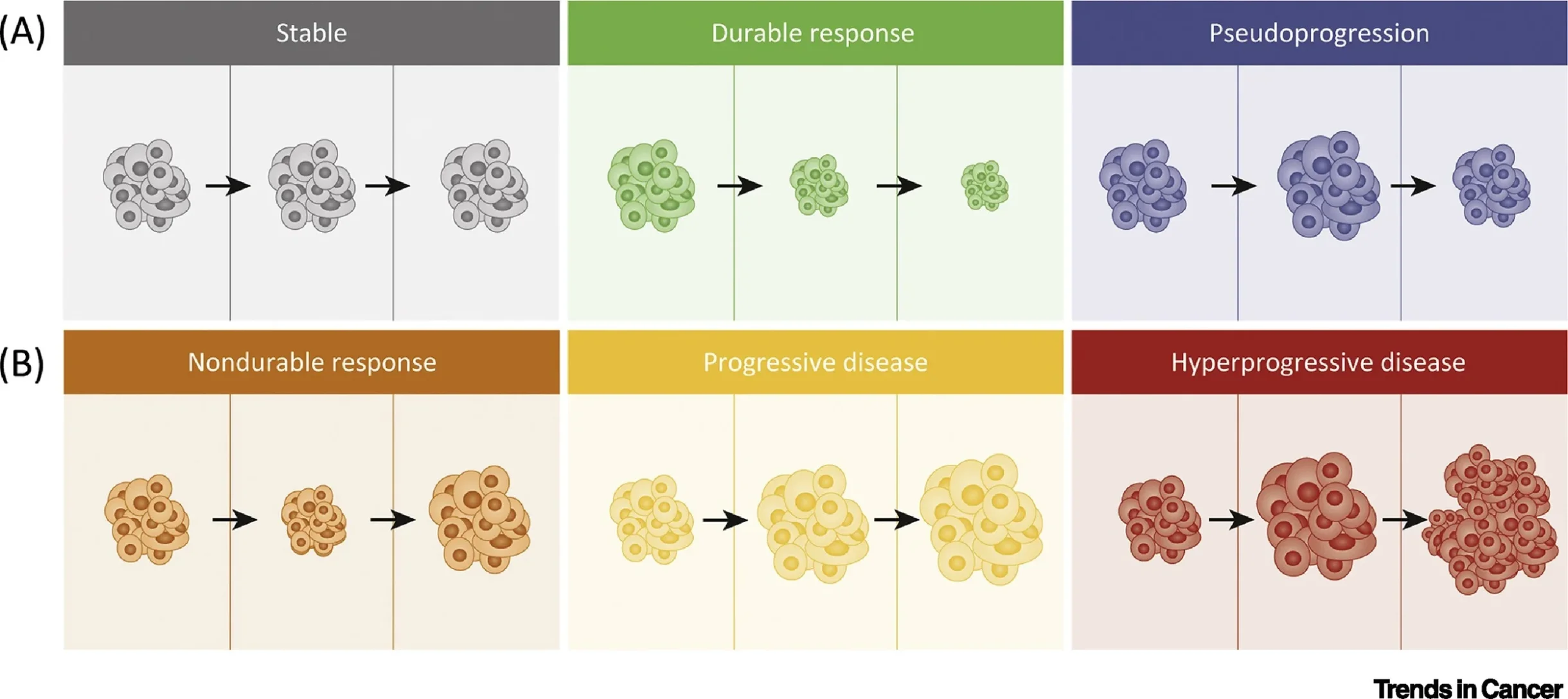
Among these, pseudoprogression remains one of the most debated and clinically consequential phenomena. It is generally defined as an initial increase in measured tumor burden and/or the appearance of new lesions followed by subsequent tumor regression without a change in therapy, reflecting immune-mediated inflammation rather than true tumor growth. In the phase 1b KEYNOTE-001 melanoma program, Hodi and colleagues formally operationalized early vs delayed pseudoprogression using immune-related criteria, underscoring that radiologic progression on RECIST may not always represent treatment failure in ICI-treated patients. Hodi et al., Journal of Clinical Oncology reported that among pembrolizumab-treated advanced melanoma patients with adequate longitudinal imaging, 7% demonstrated atypical responses, including 5% early pseudoprogression and 3% delayed pseudoprogression (definitions based on immune-related response criteria and confirmation on subsequent imaging).
Although widely recognized, pseudoprogression remains relatively uncommon, inconsistently defined across studies, and difficult to distinguish from true progression in routine clinical practice. This uncertainty directly influences whether clinicians continue treatment beyond progression, with downstream consequences for survival opportunity, toxicity exposure, sequencing of effective therapy, and healthcare costs.
Biological Mechanisms Underlying Pseudoprogression
Biological mechanisms underlying pseudoprogression
Checkpoint blockade restores cytotoxic T-cell activity within the tumor microenvironment and can trigger an early inflammatory response that transiently increases lesion size on imaging. The mechanistic foundation is typically explained by a convergence of (1) dense infiltration of CD8⁺ tumor-infiltrating lymphocytes, (2) cytokine-mediated edema and increased vascular permeability, and (3) tumor necrosis with delayed clearance of cellular debris, each of which can produce radiologic enlargement despite biologic tumor control. This framework is consistent with immune-response evaluation work in KEYNOTE-001 that documented atypical radiologic trajectories and necessitated immune-adapted interpretation beyond RECIST alone.
A particularly practical “biology insight” is that pseudoprogression represents a state where imaging is temporarily dominated by inflammation, while tumor burden may be falling at the molecular level. This is one reason the field has increasingly emphasized biomarker-integrated response assessment rather than imaging-only decisions.
Incidence Across Tumor Types
The reported frequency of pseudoprogression varies by malignancy, therapeutic regimen, and the criteria used to define it.
Across solid tumors, a systematic review and meta-analysis by Park et al., Radiology estimated an overall pseudoprogression incidence of ~6%, remaining <10% across subgroup analyses by cancer type, pseudoprogression definition, and ICI class—highlighting both the reality of the phenomenon and its overall rarity compared with true progression.
This is the clinically critical point: most RECIST progressions early on ICIs are true progression, but a small minority are not—creating a high-stakes diagnostic dilemma.
Limitations of Conventional Response Criteria
RECIST v1.1 was developed in the era of cytotoxic therapy and is built on the assumption that an increase in tumor burden reflects treatment failure. ICIs challenge this assumption, motivating immune-adapted response frameworks. The iRECIST guideline by Seymour et al., The Lancet Oncology introduced the concept of unconfirmed progression (iUPD), permitting continued therapy in clinically stable patients and requiring repeat imaging (typically 4–8 weeks) to confirm progression as iCPD.
In practice, iRECIST improves standardization in trials, but real-world implementation remains inconsistent, and imaging alone still cannot reliably discriminate pseudoprogression from true progression without clinical and biologic context.
Emerging Biomarkers and Biological Assessment
A major advance has been the use of circulating tumor DNA (ctDNA) kinetics to distinguish inflammatory enlargement from biologic failure. In a cohort study of metastatic melanoma treated with anti–PD-1 therapy, Lee et al., JAMA Oncology showed that early longitudinal ctDNA patterns could differentiate pseudoprogression from true progression: a >10-fold decrease in ctDNA within 12 weeks was associated with pseudoprogression and improved outcomes; the reported performance included 90% sensitivity and 100% specificity for predicting pseudoprogression in their dataset, with markedly better survival for patients with RECIST progression but favorable ctDNA profiles.
This work supports a modern concept: pseudoprogression is not merely a radiology curiosity—it is a case where molecular response can precede anatomic response, and blood-based biology may prevent premature discontinuation of a potentially effective ICI.
Beyond ctDNA, future response assessment is expected to integrate multidimensional immune-biologic signals, including interferon-γ–related programs and spatial immune infiltration, to move the field toward biology-driven response interpretation.
Clinical Decision-Making and Risk Balance
Because pseudoprogression is uncommon, the decision to continue immunotherapy beyond radiologic progression should be selective and anchored in clinical stability and biologic plausibility. Premature discontinuation may eliminate the chance of delayed durable benefit in true pseudoprogression, while inappropriate continuation risks cumulative immune toxicity and delayed transition to effective salvage therapy. The strongest real-world improvement in decision confidence may come from combining short-interval reassessment (per iRECIST principles) with biologic tools such as ctDNA kinetics when available.
Conclusion
Pseudoprogression represents both the promise and the complexity of immune-mediated cancer therapy. While uncommon, accurate recognition is essential to avoid premature discontinuation in patients who may ultimately achieve durable benefit, yet overestimation risks delaying effective care for true non-responders. The future of oncology therefore lies in precision response assessment that integrates radiologic findings, tumor biology, and clinical context into a unified framework for decision-making in the immunotherapy era.