The POTOMAC Phase III trial (NCT03528694), presented by Dr. Maria De Santis at the ESMO 2025 Congress in Berlin, marks a major step forward in the management of high-risk non–muscle-invasive bladder cancer (NMIBC).
This pivotal study evaluated whether adding durvalumab, an anti–PD-L1 antibody, to BCG induction and maintenance therapy could enhance outcomes in BCG-naïve, high-risk NMIBC. The results showed a significant improvement in disease-free survival, underscoring the expanding role of immunotherapy in earlier stages of bladder cancer and offering new hope for patients at high risk of recurrence.
Background
High-risk non–muscle-invasive bladder cancer (NMIBC) remains a major therapeutic challenge, as recurrence and disease progression occur in a substantial proportion of patients despite optimal local therapy. Approximately 40% of patients relapse or progress within two years of completing standard treatment, highlighting the unmet need for more durable disease control. The current standard of care—transurethral resection of bladder tumor (TURBT) followed by Bacillus Calmette-Guérin (BCG) induction and maintenance therapy—can effectively eradicate visible disease and reduce recurrence risk, yet long-term efficacy remains limited. Many patients eventually experience BCG failure, often leading to repeated treatments, increased morbidity, or the need for radical cystectomy.
Given the established role of immune activation in bladder cancer, there is growing interest in combining systemic immune checkpoint inhibitors with local intravesical immunotherapy to boost antitumor immune responses. Durvalumab, a monoclonal antibody targeting programmed death-ligand 1 (PD-L1), has demonstrated durable responses and survival benefits in advanced urothelial carcinoma. Building on this rationale, the Phase III POTOMAC trial (NCT03528694) was designed to evaluate whether adding durvalumab to standard BCG therapy could enhance disease control and reduce recurrence in patients with BCG-naïve, high-risk NMIBC following TURBT.
Methods
This randomized, open-label Phase III study enrolled 1,018 adult patients with histologically confirmed, BCG-naïve, high-risk NMIBC following complete TURBT (including patients with carcinoma in situ).
Participants were randomized 1:1:1 into three treatment arms:
- Durvalumab + BCG (induction + maintenance)
- Durvalumab + BCG (induction only)
- BCG (induction + maintenance)
Durvalumab was administered intravenously at 1500 mg every 4 weeks for 13 cycles. Intravesical BCG was given weekly for 6 weeks (induction) and then as three weekly doses at 3, 6, 12, 18, and 24 months (maintenance). Stratification was based on the presence of carcinoma in situ and papillary disease.
The primary endpoint was investigator-assessed disease-free survival (DFS) comparing D+BCG (I+M) vs BCG (I+M).
Results
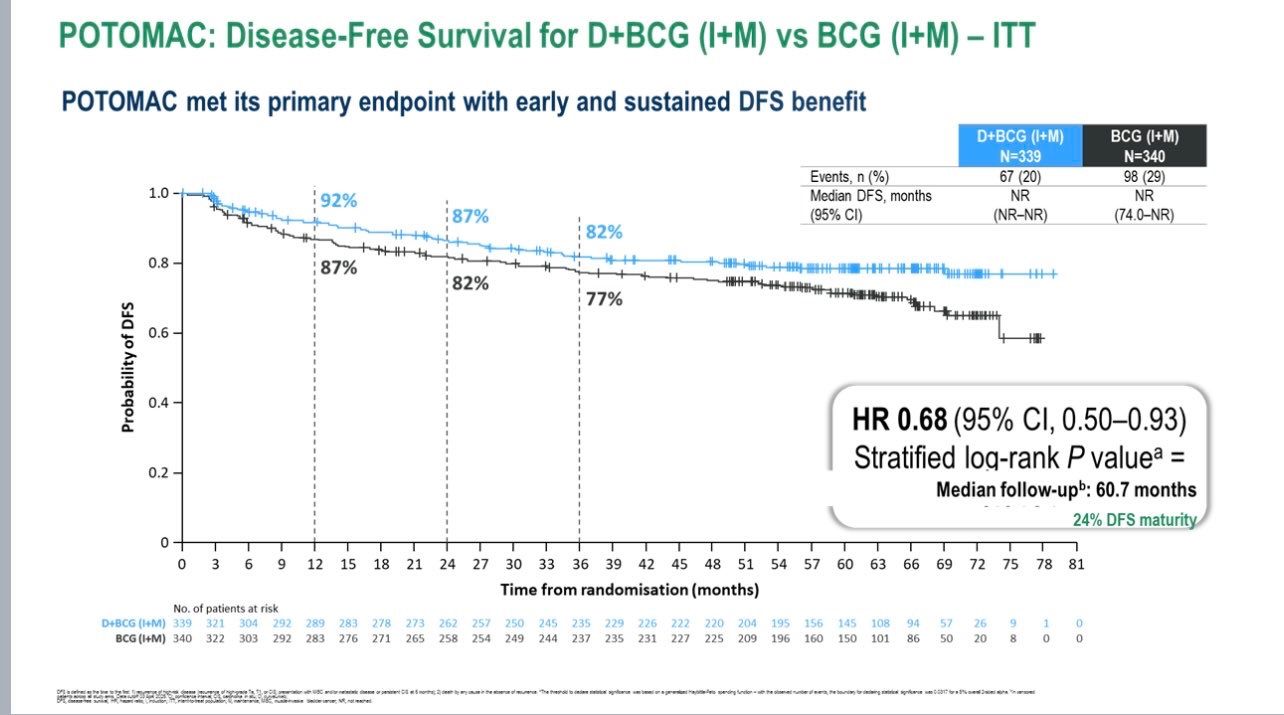
After a median follow-up of 60.7 months, the primary endpoint was met.Patients receiving durvalumab plus BCG (induction + maintenance) showed a 32% reduction in the risk of recurrence or death compared to BCG alone (HR 0.68; 95% CI 0.50–0.93; P = 0.0154).The 24-month DFS rate was 86.5% (95% CI 82.2–89.8) for D+BCG (I+M) versus 81.6% (95% CI 76.9–85.3) for BCG (I+M).
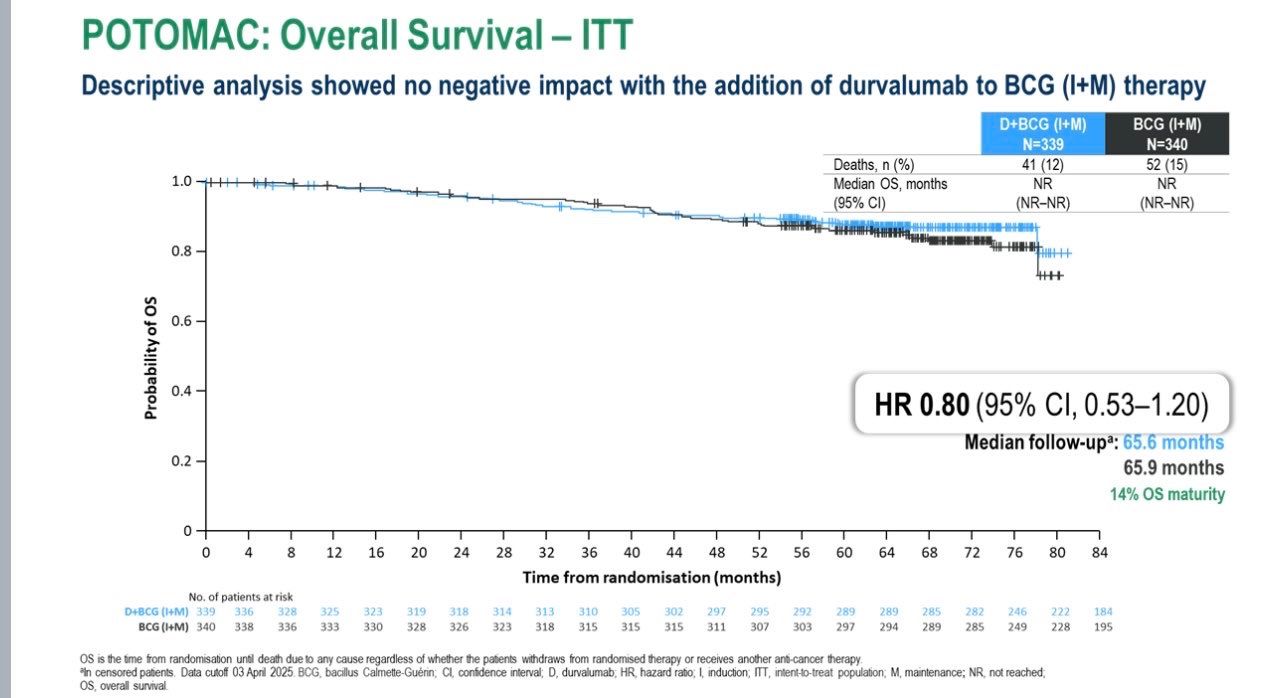
The secondary comparison—D+BCG induction only vs BCG (I+M)—did not reach statistical significance (HR 1.14; 95% CI 0.86–1.50).No detrimental effect on overall survival was observed (HR 0.80; 95% CI 0.53–1.20)>
In terms of safety, Grade 3/4 treatment-related adverse events occurred in 21% of patients receiving D+BCG (I+M), 15% in D+BCG (I), and 4% in BCG (I+M). Importantly, no treatment-related deaths were reported.
Key Insights
The combination of durvalumab with BCG demonstrated a clinically meaningful and statistically significant improvement in disease-free survival, reinforcing the potential of immunotherapy to transform outcomes in earlier stages of bladder cancer. By pairing systemic PD-L1 blockade with local immune stimulation from BCG, this strategy enhances both local and systemic antitumor immune responses — leading to deeper and more durable disease control.
Importantly, this benefit was achieved without new or unexpected safety signals, and the observed adverse events were consistent with known profiles of each agent. The manageable toxicity profile supports the feasibility of integrating this regimen into routine clinical practice.
Clinical Significance
Taken together, the POTOMAC trial establishes durvalumab plus BCG (induction + maintenance) as a potential new standard of care for patients with BCG-naïve, high-risk NMIBC, marking a pivotal step toward incorporating immune checkpoint inhibitors in non–muscle-invasive disease and expanding the scope of immunotherapy beyond advanced stages of bladder cancer.
You can read the full abstract here.


