Metaplastic breast cancer (MpBC) is a rare and aggressive form of triple-negative breast cancer (TNBC), accounting for <1% of invasive breast cancers. Characterized by glandular-to-mesenchymal or squamous differentiation, MpBC exhibits marked resistance to chemotherapy and poor prognosis compared to non-metaplastic TNBC. Historically, pathologic complete response (pCR) rates to standard neoadjuvant chemotherapy (NAC) have been as low as 2–23%, leading to debate about the role of neoadjuvant therapy in this subtype.
Given the recent success of pembrolizumab-based neoadjuvant chemo-immunotherapy (NACI) in conventional TNBC—most notably in the KEYNOTE-522 trial—this study sought to determine whether adding immunotherapy could improve outcomes in MpBC and to identify predictive biomarkers of response, particularly stromal tumor-infiltrating lymphocytes (sTILs).
Study Design
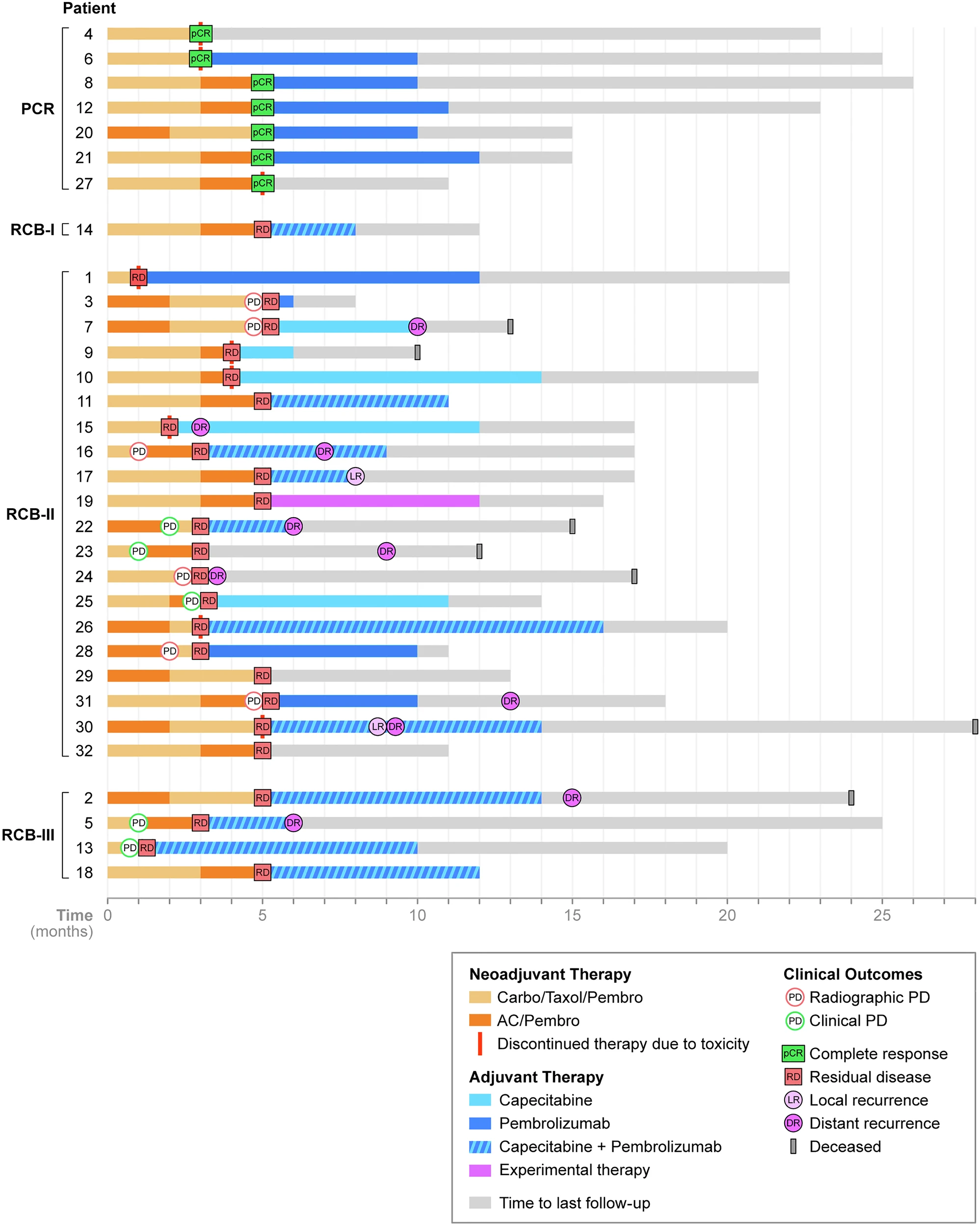
The study was a prospective institutional analysis conducted as part of the Memorial Sloan Kettering CARE-4-RARE program, which focuses on identifying biomarkers of response in rare breast cancer subtypes. A total of 32 patients with stage I–III metaplastic triple-negative breast cancer (TNBC) were enrolled and treated between July 2021 and January 2024.
All patients received neoadjuvant chemo-immunotherapy following the standard KEYNOTE-522 regimen, which included carboplatin and paclitaxel combined with pembrolizumab (CbT/Pem), followed by dose-dense doxorubicin and cyclophosphamide with pembrolizumab (AC/Pem). The primary endpoints of the study were pathologic complete response (pCR), defined as the absence of invasive cancer in both breast and lymph nodes (ypT0/Tis, ypN0), and event-free survival (EFS). Secondary analyses explored associations between treatment response and various clinicopathologic and immune biomarkers, including stromal tumor-infiltrating lymphocytes (sTILs), PD-L1 expression, tumor mutational burden (TMB), and homologous recombination deficiency (HRD) status.
Throughout neoadjuvant treatment, patients underwent frequent clinical evaluations and ultrasound monitoring to assess tumor response or progression. Following surgery, adjuvant therapy—such as pembrolizumab continuation, capecitabine, or radiation—was administered according to standard clinical practice and physician discretion.
Patient Characteristics
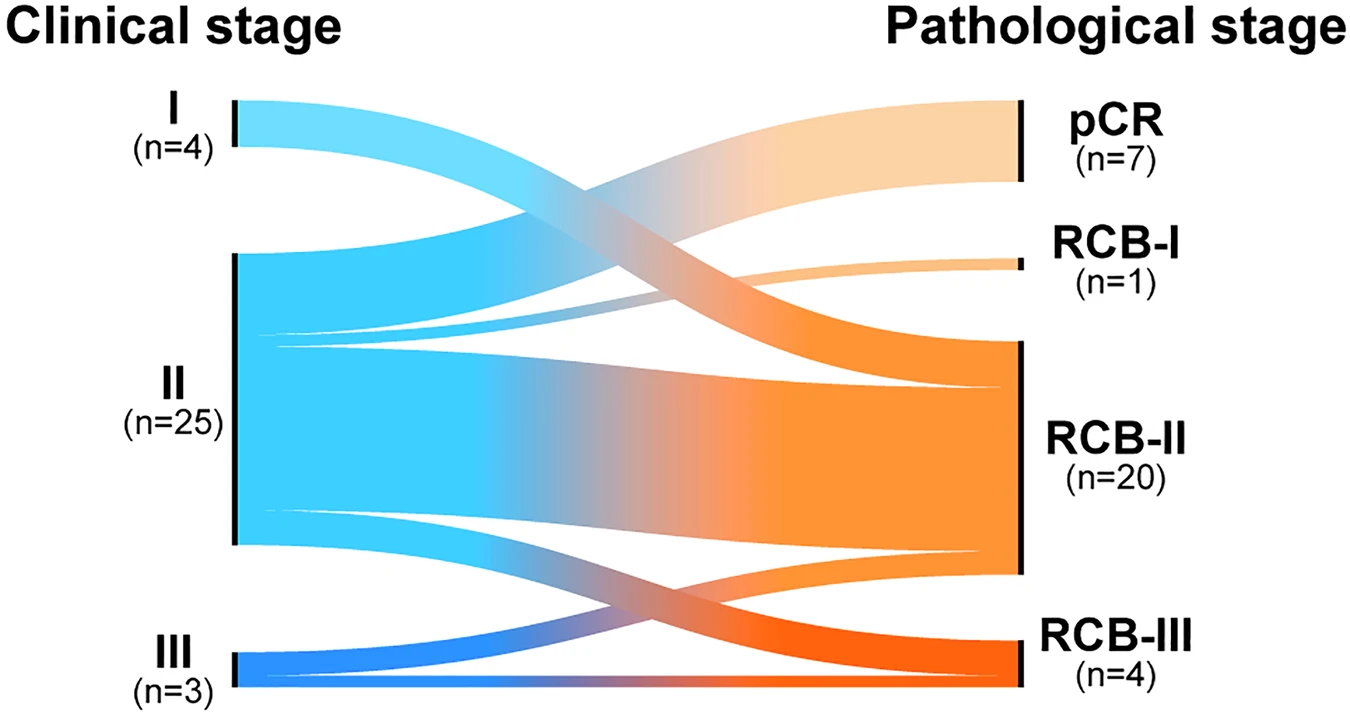
The patient cohort represented a high-risk population typically associated with poor response to chemotherapy. The median age at diagnosis was 57 years (ranging from 26 to 85 years). Most patients presented with stage II disease (78%), and a large majority (81%) were node-negative at baseline.
Histologically, the tumors demonstrated notable heterogeneity. The chondromyxoid or matrix-producing subtype was the most common, observed in 40% of cases, followed by the squamous subtype in 31%, mixed histology in 19%, and spindle cell subtype in 9% of patients. Nearly all tumors (94%) were poorly differentiated, reflecting the aggressive biology characteristic of metaplastic triple-negative breast cancer.
Germline analysis for homologous recombination deficiency (HRD)-related genes was performed in most patients, revealing pathogenic CHEK2 mutations in only two individuals, suggesting that HRD-related mechanisms were uncommon in this cohort. Overall, these clinicopathologic features underline the chemoresistant and aggressive natureof the studied population.
Results
Among the 32 patients enrolled, only 37.5% (12 patients) were able to complete the full course of neoadjuvant chemo-immunotherapy (NACI), while 34% (11 patients) experienced disease progression during treatment and 28% (9 patients) discontinued therapy early due to toxicity or personal choice. Despite these challenges, all patients remained operable and successfully proceeded to surgery.
The overall pathologic complete response (pCR) rate for the cohort was 22% (7 out of 32 patients). When analyzed by stromal tumor-infiltrating lymphocyte (sTIL) levels, the benefit of NACI was markedly greater among patients with high sTILs (≥60%), who achieved a 62% pCR rate (5/8), compared to only 9% (1/11) in those with low sTILs (p = 0.041). By histologic subtype, chondromyxoid or matrix-producing tumors accounted for 57% of all pCRs, while only 9% of patients with the squamous subtype achieved complete response. Notably, among patients with nodal involvement, 67% (4 out of 6) converted to node-negative (ypN0) status after NACI. However, the impact on breast-conserving surgery (BCS) eligibility was limited, with only one previously ineligible patient becoming eligible for BCS post-treatment.
Following surgery, adjuvant therapy was widely implemented—81% of patients received systemic treatment, including capecitabine in 16 patients and pembrolizumab continuation in 20 patients. Additionally, 93% underwent adjuvant radiation therapy as part of standard postoperative management.
At a median follow-up of 13 months, there were 2 local recurrences, 10 distant recurrences (most commonly involving the lungs and lymph nodes), and 7 deaths. Importantly, patients who achieved pCR had significantly longer event-free survival (p = 0.021) compared to those with residual disease. By 18 months, no recurrences were observed in the pCR group, whereas 66% of patients with residual disease had relapsed. The majority of distant recurrences (70%) occurred during adjuvant therapy, emphasizing the highly aggressive nature of metaplastic triple-negative breast cancer, even in the context of modern chemo-immunotherapy.
Discussion
The addition of pembrolizumab to chemotherapy achieved a modest overall pCR rate (22%), but responses were dramatically higher among tumors with high sTILs (≥60%), suggesting that an inflamed tumor microenvironmentpredicts benefit from NACI in MpBC. PD-L1 expression, however, was not correlated with pCR, reinforcing that sTILs may serve as a superior predictive biomarker in this subtype.
Notably, even though 35% of patients progressed during therapy, none became inoperable, reflecting the importance of close imaging surveillance during NACI. Furthermore, the conversion of node-positive to node-negative disease (67%) is a meaningful clinical gain that can reduce the need for axillary dissection and its associated morbidity.
The heterogeneity of MpBC, including differing immune infiltration patterns and histologic subtypes, may underlie the wide spectrum of responses—from “super-responders” to complete non-responders. Ongoing translational work using spatial transcriptomics aims to elucidate immune signatures that distinguish these groups.
Despite the modest aggregate pCR, this study demonstrates that immunogenic subsets of MpBC—especially those with high sTILs—can achieve outcomes comparable to conventional TNBC treated with NACI. Conversely, for stage IMpBC (potentially overtreated) and rapidly progressive stage III disease, NACI may not provide a survival advantage and should be carefully individualized.
Key Takeaways
- NACI with pembrolizumab yields a 22% pCR rate in metaplastic TNBC, higher than historical NAC outcomes.
- High stromal TILs (≥60%) strongly predict response (62% pCR), emerging as a potential biomarker for patient selection.
- PD-L1 expression and HRD status were not predictive of response.
- Event-free survival was significantly better in patients achieving pCR.
- Recurrences remain frequent among patients with residual disease, highlighting the need for novel post-NACI strategies, such as antibody–drug conjugates or clinical trial enrollment.
You Canc Read Article Here