The OptiTROP-Breast02 trial (NCT06081959), presented by Dr. Man Li at the ESMO Congress 2025, evaluated Sac-TMT, a novel TROP2-directed antibody–drug conjugate, in patients with hormone receptor–positive, HER2-negative metastatic breast cancer (HR+/HER2- mBC) who had progressed on CDK4/6 inhibitors and chemotherapy. Building on promising early-phase results, the study compared Sac-TMT with standard chemotherapy to assess its efficacy and safety in this heavily pretreated patient population with limited treatment options.
Background
Despite major therapeutic advances, hormone receptor–positive, HER2-negative metastatic breast cancer (HR+/HER2- mBC) remains a clinical challenge, particularly after progression on CDK4/6 inhibitors and standard chemotherapy. As resistance to endocrine therapy develops, subsequent treatment options offer limited efficacy, with modest response rates and short progression-free survival (PFS). This has created an urgent need for novel, targeted therapies capable of improving outcomes while maintaining tolerability.
TROP2, a transmembrane glycoprotein widely expressed in breast cancer cells, has emerged as a promising therapeutic target. Antibody–drug conjugates (ADCs) directed against TROP2 have shown meaningful antitumor activity in multiple solid tumors, validating this pathway as a viable strategy.
Sac-TMT is an innovative TROP2-directed ADC engineered with a novel, cleavable linker and a belotecan-derived topoisomerase I inhibitor payload, designed to enhance intracellular drug delivery and minimize systemic toxicity.
In the Phase 1/2 study (Ouyang et al., J Hematol Oncol, 2025), Sac-TMT demonstrated encouraging efficacy and a manageable safety profile in heavily pretreated HR+/HER2- mBC. Building on these findings, the Phase 3 OptiTROP-Breast02 trial (NCT06081959) was initiated to confirm the efficacy and safety of Sac-TMT compared with standard chemotherapy in this population.
Methods
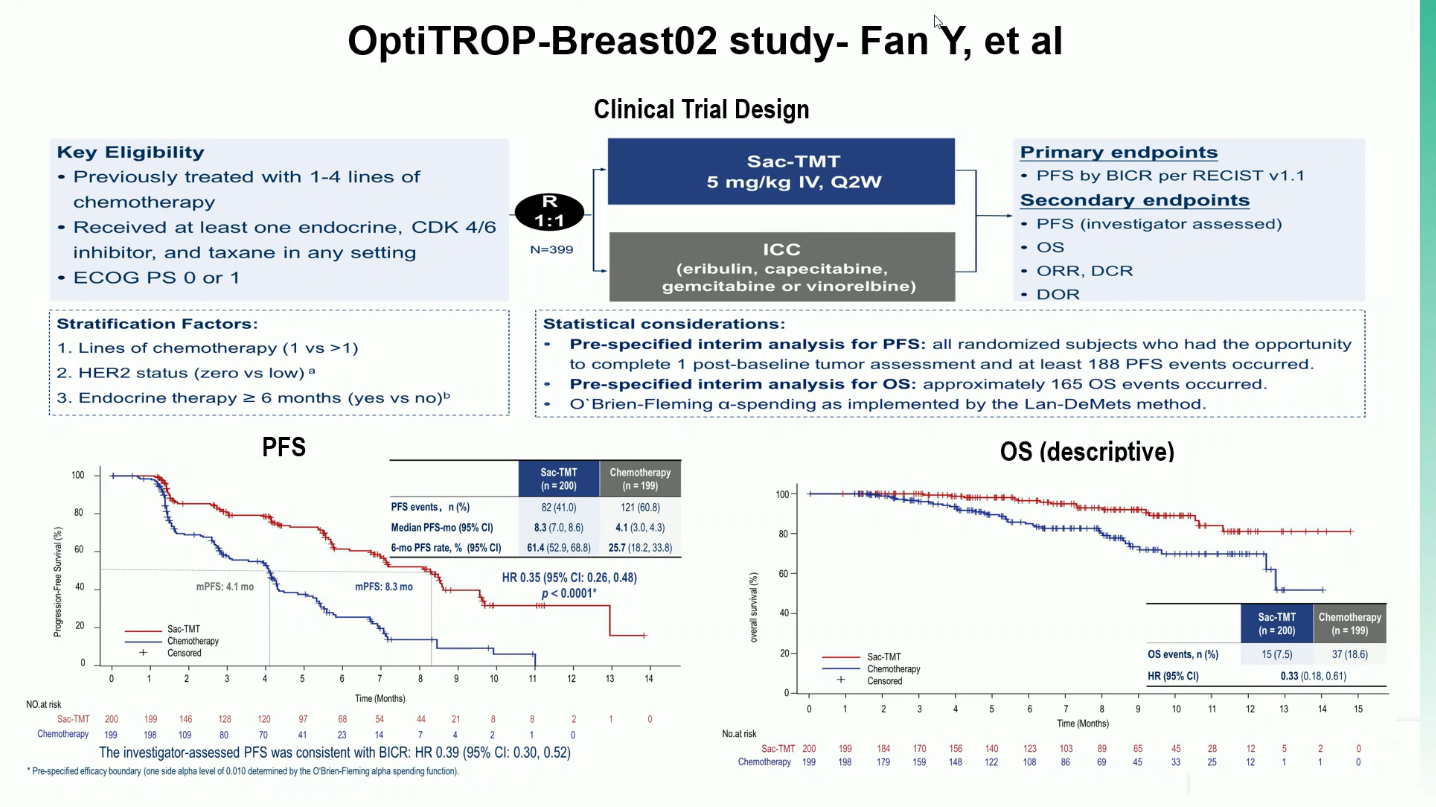
A total of 399 patients were randomized (1:1) to receive Sac-TMT 5 mg/kg every 2 weeks or investigator’s choice chemotherapy (ICC) (eribulin, vinorelbine, capecitabine, or gemcitabine). The primary endpoint was progression-free survival (PFS), assessed by blinded independent central review (BICR).
Results
The OptiTROP-Breast02 trial met its primary endpoint, showing a significant progression-free survival (PFS) benefit with Sac-TMT compared with investigator’s choice chemotherapy (ICC). Key efficacy outcomes are summarized below.
- Median PFS was 8.3 months with Sac-TMT versus 4.1 months with ICC (HR 0.35; 95% CI 0.26–0.48; P<0.0001).
- The 6-month PFS rate was 61.4% with Sac-TMT compared to 25.7% with ICC, and objective response rate (ORR) reached 41.5% vs 24.1%, respectively. Benefit was observed across both HER2-zero (HR 0.39) and HER2-low (HR 0.31) subgroups.
- Although overall survival (OS) data remain immature, a favorable trend was seen with Sac-TMT (HR 0.33; 95% CI 0.18–0.61).
- Grade ≥3 treatment-related adverse events occurred in 62.0% of patients on Sac-TMT and 64.8% on ICC, most commonly neutropenia and leukopenia. Treatment discontinuations were rare (0% vs 0.5%), and pneumonitis was infrequent (1.5% vs 1.0%, all grade 1–2).
Conclusion
The OptiTROP-Breast02 trial demonstrated that Sac-TMT delivers a clinically meaningful and statistically significant improvement in progression-free survival compared with standard chemotherapy, establishing its therapeutic relevance in the management of pretreated HR+/HER2- metastatic breast cancer.
The efficacy benefit was consistent across HER2-low and HER2-zero subgroups, reinforcing Sac-TMT’s potential to address an unmet need in this heterogeneous population. Importantly, the ADC exhibited a manageable safety profile, with low treatment discontinuation rates and limited high-grade toxicities, underscoring its favorable risk–benefit balance. Collectively, these findings position Sac-TMT as a promising new treatment option for patients who have exhausted standard endocrine and chemotherapy regimens, potentially expanding the role of TROP2-targeted therapies in breast cancer care.
You can read the full abstract here.


