Immune checkpoint inhibitors (ICIs) are a type of immunotherapy that enhances the body’s immune response against cancer cells by targeting specific proteins on immune cells, such as T cells, or on tumor cells. These proteins, known as immune checkpoints, normally prevent the immune system from attacking healthy cells by sending “off” signals. Immune checkpoint inhibitors block these checkpoints, allowing T cells to recognize and destroy cancer cells more effectively. Immune checkpoint inhibitors are used to treat various cancers, including melanoma, lung cancer, renal cell carcinoma, and more. They are particularly effective in cancers with high PD-L1 expression or DNA repair deficiencies.
Immune checkpoint inhibitors are a powerful class of immunotherapy that enhance the body’s ability to fight cancer but can also trigger immune-related adverse events (irAEs), including ocular toxicities. While less common than systemic irAEs, ocular side effects can affect various structures of the eye, leading to conditions such as dry eye syndrome, uveitis, episcleritis, scleritis, optic neuritis, and retinopathy.
In this article, you will learn about the ocular side effects of immune checkpoint inhibitors, including how to diagnose, manage, and prevent these complications.
Historical Timeline of Immune Checkpoint Inhibitors (ICIs)
The development of immune checkpoint inhibitors (ICIs) represents a major breakthrough in cancer therapy, building on over a century of immunotherapy research. In 1891, William B. Coley pioneered cancer immunotherapy with bacterial injections, known as Coley’s Toxins, though this approach eventually fell out of favor.
Decades later, key immune checkpoints were discovered, laying the foundation for modern ICIs. The CTLA-4 gene, a negative regulator of T-cell activity, was identified in 1987, followed by the discovery of PD-1 in 1992. By the early 2000s, researchers understood that tumors exploit PD-L1 to suppress T-cell responses, paving the way for PD-1/PD-L1-targeted therapies.
The first major clinical breakthrough came in 2010 with the approval of sipuleucel-T (Provenge), an immune cell therapy for prostate cancer, though its impact was limited. In 2011, the FDA approved ipilimumab (Yervoy), the first CTLA-4 inhibitor, for metastatic melanoma, marking the beginning of modern immune checkpoint therapy. This was followed in 2014–2015 by the approval of PD-1 inhibitors pembrolizumab (Keytruda) and nivolumab (Opdivo) for melanoma and other cancers.
A significant milestone occurred in 2017 when the FDA granted the first tissue-agnostic approval for pembrolizumab, allowing its use based on molecular markers rather than tumor location. This marked a shift in cancer treatment, highlighting the growing role of ICIs in precision oncology.
How Do Immune Checkpoint Inhibitors Work?
ICIs help the immune system fight cancer by blocking signals that tumors use to avoid detection. These drugs target PD-1, PD-L1, and CTLA-4, which normally prevent excessive immune responses. PD-1 is a receptor on T cells that reduces their activity when it binds to PD-L1 or PD-L2. Tumors exploit this by producing PD-L1, which shuts down T cells and helps cancer grow. Blocking PD-1 or PD-L1 with ICIs reactivates T cells, allowing them to attack the tumor.
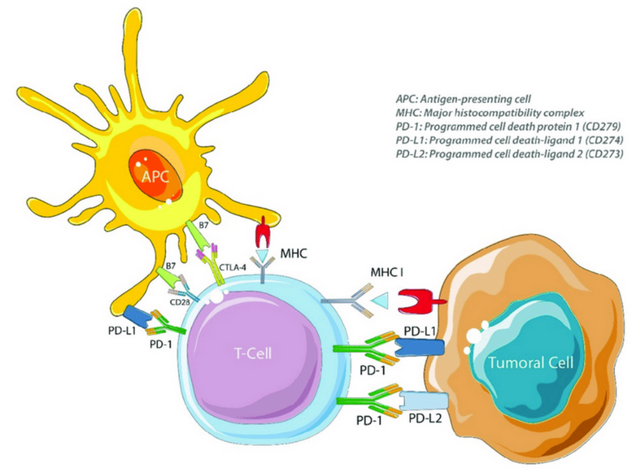
PD-1, PD-L1 and CTLA-4 targets of immune checkpoint inhibitors. APC = antigenpresenting cell, CD28 = cluster of differentiation 28, CTLA-4 = cytotoxic T lymphocyte antigen 4, MHC = major histocompatibility complex, MHC I = major histocompatibility complex class I, PD-1 = programmed cell death protein 1, PD-L1 = programmed cell death 1 ligand 1 and PD-L2 = programmed cell death 1 ligand 2.
CTLA-4 is another checkpoint that limits T-cell activation early in an immune response. Tumors use this to weaken the immune system. ICIs that block CTLA-4 boost T-cell activity, improving the body’s ability to fight cancer but also increasing the risk of immune-related side effects.
While ICIs have transformed cancer treatment, their ability to enhance immune responses can also cause the immune system to attack healthy tissues, requiring careful management.
Which Cancer Types Are Treated With Immune Checkpoint Inhibitors?
ICIs are widely used in cancer treatment, offering significant benefits across various malignancies. In melanoma, ICIs such as ipilimumab and pembrolizumab are first-line treatments for advanced disease. Weber J, N Engl J Med 2017. Non-small cell lung cancer (NSCLC) is another major indication, with drugs like atezolizumab and pembrolizumab recommended as first-line therapies. Renal cell carcinoma (RCC) also responds well to ICIs, particularly in combination with other agents.
ICIs play a crucial role in bladder cancer, where they are approved for treating urothelial carcinoma. In head and neck squamous cell carcinoma (HNSCC), pembrolizumab is used for recurrent or metastatic cases. For colorectal cancer (CRC), ICIs are effective in tumors with microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR) characteristics.
The use of ICIs has expanded to liver cancer, where they are included in treatment regimens, and cervical cancer, with pembrolizumab approved for recurrent or metastatic cases. These therapies continue to transform cancer treatment by enhancing the immune system’s ability to target tumors.
How Do Immune Checkpoint Inhibitors Affect Ocular Health?
Immune checkpoint inhibitors cause immune dysregulation by blocking inhibitory signals that normally regulate T-cell activation, leading to an unchecked immune response. ICIs target molecules like CTLA-4, PD-1, and PD-L1, which are crucial for maintaining immune tolerance. This dysregulation can trigger immune-related adverse events (irAEs) in the eye, such as uveitis, dry eye, and retinopathy. The eye’s immune-privileged status, maintained by molecules like PD-1, is compromised when these pathways are blocked, increasing the risk of autoimmune reactions.
T-cell activation plays a central role in ocular inflammation induced by ICIs. Th1 cells release interferon-gamma (IFN-γ), a cytokine implicated in uveitis pathogenesis, while Th17 cells produce interleukin-23 (IL-23), promoting autoimmune responses. Blocking PD-1 or CTLA-4 amplifies T-cell-mediated inflammatory pathways, leading to conditions like uveitis, dry eye syndrome, and intraocular inflammation. These effects are more pronounced with CTLA-4 inhibitors due to their upstream impact on T-cell initiation compared to PD-1 inhibitors.
Immune Checkpoint Inhibitors Associated Ocular Side Effects
Ocular side effects of Immune Checkpoint Inhibitors can be clinically significant. These irAEs are thought to result from immune system overactivation, leading to inflammation in ocular structures. Reported toxicities range from mild dry eye syndrome to severe vision-threatening conditions such as uveitis, optic neuritis, and retinopathy.
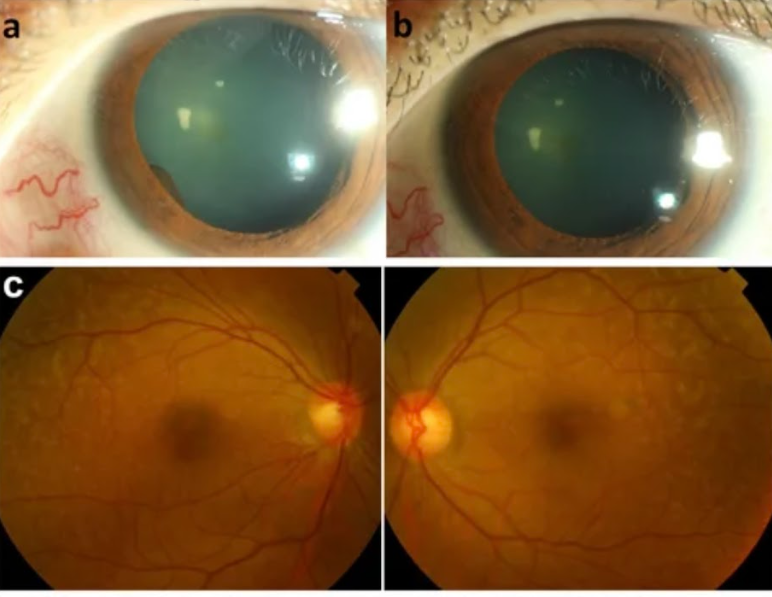
Photograpgh of the anterior eye. (A) an iris cyst was found 1 month after receiving immunotherapy with pembrolizumab. (B)resolution of anterior chamber cells and iris cysts after topical prednisolone and cycloplegics for 2 weeks. Fundus photography of fundus lesions in the bilateral eyes. (C) chorioretinal lesions with superotemporal macular mottling can be seen. Fundus autofluorescence (FAF) of fundus lesions in the bilateral eyes.
The Most Common Types Of Ocular Side Effects
ICIs can cause dry eye disease, uveitis, Vogt-Koyanagi-Harada (VKH) disease, and neuro-ophthalmic issues like optic neuritis and myasthenia gravis. Early detection and treatment with corticosteroids or immunosuppressants are crucial.
Dry Eye Syndrome
Dry eye disease, characterized by tear hyperosmolarity, tear-film instability, and ocular surface inflammation, results from decreased tear quantity or quality. It is the most frequently reported ocular side effect associated with immune checkpoint inhibitors. Patients often present with nonspecific eye irritation, and it may occur as part of sicca syndrome. In severe cases, it can lead to corneal perforation. ICI-related dry eye is typically managed with artificial tears and topical cyclosporine.
Uveitis
Among immune checkpoint inhibitor related ophthalmic toxicities, uveitis is one of the most frequently reported ocular side effects. It has been associated with anti-CTLA-4 and anti-PD-1/PD-L1 agents, including ipilimumab, durvalumab, avelumab, pembrolizumab, nivolumab, and atezolizumab. The overall risk for uveitis significantly increases after 1 year, and a strong association is linked to nivolumab, a PD-1 inhibitor, which accounted for 32% of total uveitis cases but is most strongly associated with ipilimumab, a CTLA-4 inhibitor. Fang, T. J Curr Ophthalmol 2019
ICI-related uveitis can present as anterior, posterior, or pan-uveitis. The onset varies widely, ranging from 2 weeks to 14 months after the initial ICI infusion. Patients typically report conjunctival redness or bilateral blurred vision as initial symptoms, though eye pain, photophobia, and floaters are also common.
Management involves periocular, topical, or systemic corticosteroids to control inflammation, while intravitreal corticosteroids may be used for macular edema .
Notably, ICI therapy, particularly anti-PD-1 agents, has been linked to Vogt-Koyanagi-Harada (VKH) disease, a bilateral diffuse granulomatous uveitis. VKH presents with blurry vision, xanthopsia, and exudative retinal detachments accompanied by bilateral uveitis. Treatment typically involves systemic corticosteroids, with occasional use of topical corticosteroids. Kikuchi, R, BMC Ophthalmol 2020
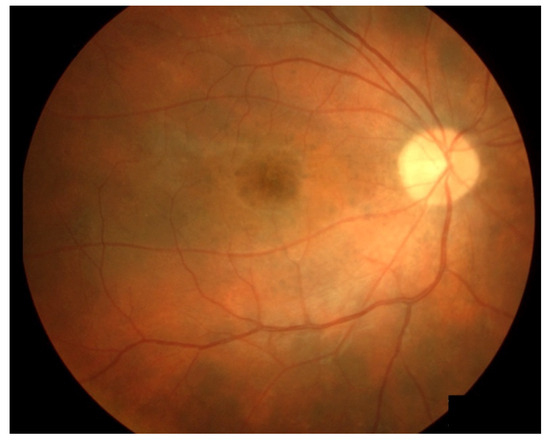
Fundus photographs of a VKH patient who developed “sunset glow fundus”. From Hirooka et al., 2017
Neuro-Ophthalmic Side Effects
Neurologic complications such as optic neuritis, Graves’ orbitopathy, papillitis, and myasthenia gravis have been documented, with a median onset of 35 days after initiating ICI therapy. Harrison RA, Curr Neurol Neurosci Rep. 2020
Additional reported manifestations include papilledema, multiple cranial neuropathies, and cerebellar ataxia with associated nystagmus.
The severity of neuro-ophthalmic side effects varies, ranging from mild visual disturbances to significant vision loss, disc edema, and scotomas. Early recognition and prompt management are critical to preventing long-term complications. While most cases respond to corticosteroids, severe optic neuropathy may require intravenous immunoglobulin or plasmapheresis.
Rare Ocular Side Effects Of Immune Checkpoint Inhibitors
ICIs can cause retinal detachment, conjunctivitis, ocular myasthenia gravis, thyroid-like orbitopathy, and inflammatory orbitopathy.
Retinal Detachment
Uveal effusion has been reported in patients receiving anti-PD-1/PD-L1 agents, including pembrolizumab, nivolumab, and atezolizumab. ICI-associated uveal effusion typically presents with symptoms such as redness, blurry vision, and ocular discomfort, often accompanied by serous choroidal detachment with intraretinal and subretinal fluid, including foveal involvement. While intraocular inflammation can also contribute to uveal effusion, its resolution following ICI discontinuation suggests a direct role of ICIs in its development.
There are also isolated reports of serous retinal detachment, with or without choroidopathy, immune retinopathy, exudative retinal detachment with ciliochoroidal effusion, and melanoma-associated retinopathy with atypical chorioretinal lesions. These conditions often lead to photophobia and blurry vision and have been managed using various approaches, including topical and oral corticosteroids, ICI discontinuation, or observation alone.
Conjunctivitis
Conjunctivitis is a rare ocular side effect in patients receiving immune checkpoint inhibitors, with variable incidence and time to onset.
Ophthalmologic evaluation confirmed bilateral sterile conjunctivitis without retinal or uveitic involvement, and partial symptom relief was achieved with topical steroid therapy.
Orbital Side Effects
Ocular myasthenia gravis is the most frequently reported immune checkpoint inhibitor related ocular side effect affecting the orbit and ocular adnexa. It can manifest as blepharoptosis, ocular motility disturbances, diplopia . Management includes oral or intravenous corticosteroids, intravenous immunoglobulin, pyridostigmine, and plasmapheresis.
Thyroid-like Orbitopathy
Thyroid-like orbitopathy is a rare immune-mediated inflammatory disorder affecting the ocular and orbital tissues. It has been infrequently reported as an adverse event, with ophthalmoplegia and proptosis being the most commonly observed symptoms. While this condition is primarily associated with CTLA-4 inhibitors, cases have also been documented in patients receiving nivolumab, pembrolizumab, and combination ICI therapy. Sagiv, O. Ophthalmic Plast Reconstr Surg 2019.
Inflammatory Orbitopathy
Inflammatory orbitopathy has been primarily reported in association with ipilimumab and pembrolizumab therapy and is typically managed with high-dose corticosteroids. Nardin, C. Invest. New Drugs 2019 . Recognizing inflammatory orbitopathy as a distinct entity from thyroid-associated orbitopathy is crucial when evaluating ocular inflammatory conditions related to ICI therapy.
How Can We Diagnose Ocular Side Effects of Immune Checkpoint Inhibitors?
Patients experiencing ophthalmic immune-related adverse events (irAEs) require comprehensive ophthalmologic assessments to evaluate the presence of ocular, fundus, neurologic, and systemic conditions.
The investigation of neuro-ophthalmic events may involve a range of imaging and laboratory tests based on the patient’s medical history and examination findings, including infectious and autoimmune serologies, MRI, optical coherence tomography (OCT), and cerebrospinal fluid (CSF) analysis. Orbital and cerebral MRIs can offer valuable diagnostic and prognostic data for ophthalmologists.
During ophthalmologic evaluation, a significant decrease in visual acuity was noted, along with non painful redness in both eyes. A slit-lamp examination showed strong positive anterior chamber cells, bilateral granulomatous keratic precipitates, bilateral anterior and posterior synechiae, and some pigmentary deposits on the anterior lens capsule, predominantly in the left eye. Fluorescein angiography confirmed unilateral or bilateral papilledema, while OCT revealed mild macular edema and a subfoveal serous retinal detachment.
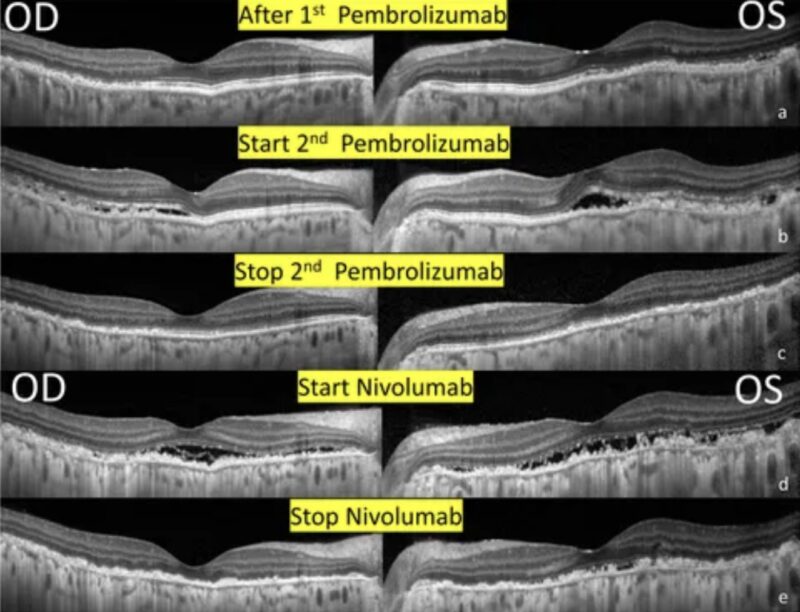
Serial changes in optical coherence tomography of the bilateral eyes. (A) 1 month after receiving immunotherapy with pembrolizumab. Note the RPE and outer retinal destruction over the heterogeneous changes of the superior and temporal region. (B) 1 week after receiving 2nd Pembrolizumab. Multiple subretinal fluids with RPE and outer retinal destruction involving the fovea developed. (C) After the discontinuation of pembrolizumab. A resolution of SRF but extensive RPE and outer retina destruction remained the same. (D) After receiving immunotherapy with nivolumab. New subretinal fluids with more prominent RPE and outer retinal destruction involving the fovea. (E) After the discontinuation of Nivolumab. A resolution of SRF and sustained extensive RPE and outer retina destruction.
What Is The Management Of Ocular Side Effects?
Ophthalmic toxicities can be managed with periocular, topical, or systemic corticosteroids in the initial stages. In cases where ocular inflammation is mild or limited to the anterior segment, discontinuing ICIs may not be necessary. However, decisions regarding treatment cessation or continuation should be made on a case-by-case basis, depending on the severity of symptoms and available ophthalmologic treatments.
In severe or steroid-refractory cases, where symptoms are debilitating, collaboration with oncologists is crucial to weigh the benefits and risks of continuing or halting ICI therapy. Although spontaneous resolution of symptoms after stopping immunotherapy is rare, steroids are commonly used to manage the duration and intensity of symptoms. For patients with severe ophthalmic events, alternative treatments such as intravenous immunoglobulin or plasma exchange may be beneficial.
You Can Also Read Ocular Side Effects of Chemotherapy: Understanding the Risks of Eye Toxicity by OncoDaily

Optimizing Immune Checkpoint Inhibitor Therapy: Ophthalmologist-Oncologist Collaboration
Collaboration between ophthalmologists and oncologists is essential for preventing and managing ocular side effects associated with immune checkpoint inhibitors (ICIs). This multidisciplinary approach ensures that both systemic cancer treatment and ocular health are carefully monitored and managed. Ophthalmologists can perform baseline eye examinations, detect early signs of immune-related ocular toxicity (e.g., uveitis, dry eye, optic neuritis), and provide timely interventions such as corticosteroid therapy or immunosuppressive treatments.
Oncologists, in turn, can evaluate the overall impact of ICIs on the patient’s immune system and collaborate with ophthalmologists to modify treatment plans if significant ocular complications occur. Regular communication between both specialists helps optimize treatment outcomes, minimize vision-related side effects, and maintain the patient’s quality of life throughout immunotherapy.
You Can Also Watch Dr. Jason Luke’s Journey in Immuno-Oncology Innovation by OncoDaily
Written by Margarita Pozlikyan MD
FAQ
What are the most common ocular side effects of ICIs?
The most frequently reported ocular immune-related adverse events (irAEs) include dry eye, uveitis, and conjunctivitis. Ophthalmoplegia and keratitis are also notable.
How often do ocular side effects occur?
Ocular irAEs are relatively uncommon, occurring in approximately 1-3% of patients treated with ICIs.
Which ICIs are associated with ocular side effects?
Ocular irAEs have been reported with the use of Ipilimumab, Nivolumab, Pembrolizumab, Atezolizumab, Avelumab, and Durvalumab.
What are the symptoms of ICI-induced dry eye?
Symptoms include foreign body sensation, red eye, irritation, blurry vision, and photophobia. These can appear days to months after starting ICI therapy.
Are ocular irAEs more common in specific cancers?
Yes, ocular irAEs are more strongly associated with melanoma and lung cancer, with uveitis and retinal disorders being more prevalent in melanoma patients.
How are ocular irAEs managed?
Most cases are mild to moderate and resolve with conservative treatment or corticosteroids. Early ophthalmic examination is crucial to prevent permanent vision loss.
Can ocular irAEs lead to treatment discontinuation?
In some cases, ocular irAEs contribute to the decision to discontinue ICI therapy, especially if symptoms are severe or persistent.
How Do Immune Checkpoint Inhibitors Work?
Immune checkpoint inhibitors are a type of immunotherapy that helps the immune system recognize and attack cancer cells. They work by blocking proteins called checkpoints, which are part of the immune system's normal function to prevent it from attacking healthy cells. By blocking these checkpoints, such as PD-1 or PD-L1, the inhibitors allow the immune system to target and destroy cancer cells more effectively.
What Are the Common Side Effects of Immune Checkpoint Inhibitors?
Common side effects of immune checkpoint inhibitors include diarrhea, fatigue, cough, nausea, skin rash, and muscle and joint pain. More serious side effects can occur, such as autoimmune reactions leading to inflammation in various organs like the lungs, liver, or kidneys. It is crucial to report any new side effects to healthcare providers promptly
How Important is Communication Between Oncologists and Ophthalmologists?
Effective communication between oncologists and ophthalmologists is crucial for managing ocular toxicities associated with cancer treatments. This collaboration ensures that patients receive comprehensive care, especially when dealing with side effects like dry eye or uveitis from immune checkpoint inhibitors. Regular updates and open dialogue help in early detection and management of ocular complications.
What Are the Benefits of Collaborative Care in Ophthalmic Oncology?
Collaborative care between oncologists and ophthalmologists offers several benefits, including improved patient outcomes, better management of ocular side effects from cancer treatments, and enhanced access to specialized care. This collaboration also fosters research and innovation in ocular oncology, leading to more effective treatments for eye cancers and related conditions


