Immunotherapy is revolutionizing medicine by leveraging the immune system to fight diseases, especially cancer. It includes treatments like monoclonal antibodies, immune checkpoint inhibitors, CAR T-cell therapy, and therapeutic vaccines. CAR T-cell therapy, in particular, has shown strong results in blood cancers like leukemia and lymphoma. It works by modifying a patient’s T cells to target cancer cells directly. Despite its success, it can cause serious side effects, including cytokine release syndrome (CRS) and neurotoxicity (ICANS), requiring close monitoring. In addition to these systemic effects, localized toxicities such as ocular side effects are gaining attention.

Photo:Depositphotos
In this article, you will learn about ocular side effects of CAR-T cell therapy. It also explains how these side effects might show up and what can be done to manage them.
What Is CAR T-Cell Therapy?
CAR T-cell therapy is a groundbreaking form of immunotherapy that involves genetically modifying a patient’s T cells to recognize and eliminate cancer cells. This is done by engineering the T cells to express Chimeric Antigen Receptors (CARs)—synthetic fusion proteins that enable the T cells to specifically bind to antigens found on the surface of tumor cells.
Each CAR typically includes three key components: an extracellular domain, usually a single-chain variable fragment (scFv), which recognizes a tumor-specific antigen; a transmembrane domain that anchors the receptor to the T cell membrane; and an intracellular signaling domain that activates the T cell upon antigen binding. Modern CARs also include co-stimulatory domains such as CD28 or 4-1BB to enhance T cell activation, persistence, and effectiveness.
In clinical practice, T cells are harvested from the patient’s blood, genetically modified in a laboratory to express CARs that target a specific cancer antigen (such as CD19 in certain blood cancers), and then expanded and infused back into the patient. Once administered, these engineered T cells locate and destroy cancer cells through direct cytotoxicity, induction of apoptosis, and cytokine release that recruits other immune cells. Because each cancer type expresses different antigens, CARs are specifically designed for the target cancer.
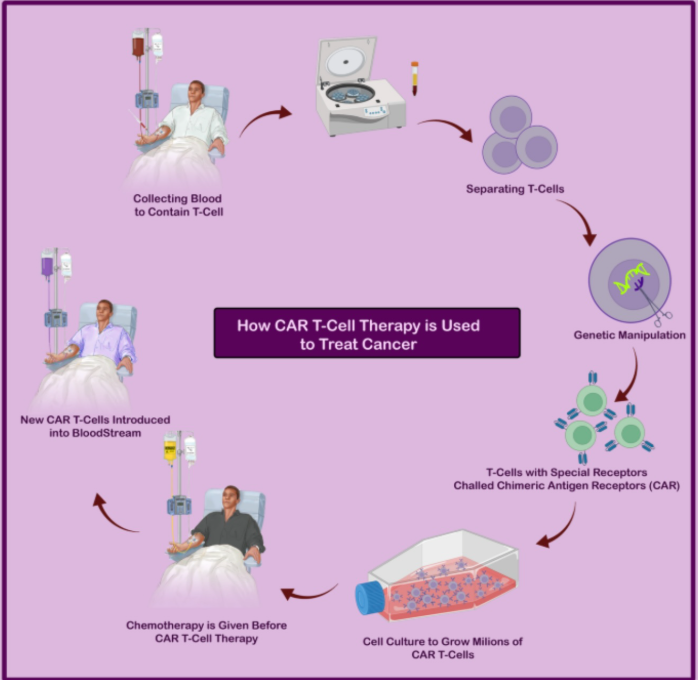
Several CAR T-cell therapies have received U.S. Food and Drug Administration (FDA) approval for blood cancers, including certain lymphomas, leukemias, and multiple myeloma. These include Kymriah (approved in 2017), Yescarta (2017), Tecartus (2020), Breyanzi (2021), Abecma (2021), and Carvykti (2022). Ongoing clinical trials continue to explore next-generation CAR T therapies and strategies to extend their use to solid tumors, aiming to improve outcomes for a wider range of cancer patients.
Which Antigens Are Targeted in Different CAR T Therapies?
CAR T-cell therapies are genetically engineered to recognize and attack specific antigens found on the surface of cancer cells, offering a highly targeted approach to treating various malignancies. One of the most extensively studied targets is CD19, commonly expressed in B-cell cancers such as acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL); approved therapies include Kymriah and Yescarta. In multiple myeloma, the main target is BCMA (B-cell maturation antigen), with effective treatments like Abecma and Carvykti.
Other promising targets include CD20, also found in B-cell malignancies; CD30, used in the treatment of refractory Hodgkin’s lymphoma; and CD33, CD123, and FLT3, which are under investigation for acute myeloid leukemia (AML). In the realm of solid tumors, ganglioside GD2 is being explored in neuroblastoma and certain other cancers, while FAP (fibroblast activation protein) and VEGFR-2 (vascular endothelial growth factor receptor 2) are being investigated for their role in targeting the tumor stroma and vasculature.
CAR T cell therapy in Multiple myeloma (MM), Photo:Depositphotos
What Are the Common Side Effects of CAR-T Cell Therapy?
Most side effects from CAR-T therapy occur within the first few days after treatment, but some may appear up to eight weeks later.
Possible side effects include cytokine release syndrome (a strong immune reaction), neurological problems such as confusion or seizures, tumour lysis syndrome caused by the rapid breakdown of cancer cells, and allergic reactions. CAR-T therapy can also lower your B cell count, which may increase your risk of infection.
Cytokine Release Syndrome
Cytokine Release Syndrome (CRS) is a clinical condition caused by widespread immune activation following CAR-T cell therapy. It is associated with the rapid expansion of CAR-T cells and a marked increase in cytokines and other inflammatory markers in the blood. CRS typically occurs within 1 to 14 days after infusion, depending on the specific CAR-T product, clinical trial design, and patient population. The first signs of CRS are usually fever, myalgias, fatigue, and general discomfort. Fevers may start as low-grade but can escalate over several days, often exceeding 40.5°C.
Additional symptoms may include chills, dizziness from low blood pressure, difficulty breathing, headaches, and hypoxia. In more severe cases, CRS can progress to life-threatening complications such as vasodilatory shock, capillary leak syndrome, and organ dysfunction. The severity of CRS can vary—some cases are self-limited and respond well to supportive care like antipyretics and intravenous fluids, while others require treatment with anti-cytokine therapies such as tocilizumab or corticosteroids. In the most serious cases, patients may need care in the Intensive Care Unit (ICU). With appropriate monitoring and intervention, CRS typically resolves within two to three weeks after treatment.
Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS)
Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) is a frequent neurological complication of CAR-T cell therapy. ICANS is associated with elevated cytokine levels in the cerebrospinal fluid and disruption of the blood-brain barrier. Santomasso BD, Cancer discovery 2018. It can occur with all FDA-approved CAR-T products, indicating that it is not specific to any single antigen target such as CD19.
Patients at higher risk for developing ICANS include those who are younger, have pre-existing neurological or medical conditions, high tumor burden, receive intensive lymphodepleting chemotherapy, experience early or severe cytokine release syndrome (CRS), or have significant cytopenias.
Clinically, ICANS can range from mild symptoms such as confusion, headache, attention deficits, and language disturbances (including word-finding difficulties) to severe manifestations such as seizures, transient coma, focal neurological deficits, or life-threatening cerebral edema. Lee DW, J Am Soc Blood Marrow Transplant, 2019. Symptoms often begin with inattention and language impairment and can progress rapidly over hours to days.
What Are The Ocular Side Effects Of CAR-T Cell Therapy?
While CAR-T-cell therapy has demonstrated remarkable systemic efficacy in the treatment of hematologic malignancies, its role in ocular involvement—either from malignant infiltration or as a therapeutic strategy for primary intraocular tumors—remains relatively underexplored. Preclinical studies have shown encouraging results, suggesting that CAR-T cells may have potential ophthalmic applications. For instance, CAR-T cells targeting CD171 and GD2 have achieved near-complete eradication of retinoblastoma cell lines in vitro. Similarly, HER2-targeted CAR-T cells have demonstrated antitumor activity in in vitro assays and animal models of uveal melanoma.
A major barrier to the ocular application of CAR-T therapy lies in overcoming the immune-privileged status of the eye. Structures such as the blood-retina and blood-aqueous barriers significantly limit the ability of circulating immune cells and therapeutic agents to access intraocular tissues, posing a challenge for effective drug delivery. Although ocular side effects are relatively uncommon compared to systemic toxicities, several ophthalmic side effects have been reported in association with CAR-T therapy. These include new-onset visual disturbances, ocular graft-versus-host disease, herpes zoster ophthalmicus, and suspected acute retinal necrosis. Mumtaz AA, Br J Ophthalmol, 2023.
Cases of exudative retinal detachment have been observed, likely secondary to systemic inflammatory syndromes induced by CAR-T therapy. More severe manifestations, such as bilateral retinal detachment, leukemic infiltration of the retina and optic nerve, and cytomegalovirus (CMV) retinitis with subsequent retinal detachment, have also been documented, particularly in patients with multiple myeloma. Zu C, Curr Oncol. 2022. Papilledema has been reported in the context of CAR-T-associated encephalopathy. Garcia-Robledo JE, Hematol Rep.2022.
One particularly concerning ocular complication is optic neuropathy, which may be linked to immune effector cell-associated neurotoxicity syndrome (ICANS). Though rare, optic neuropathy reflects the broader neurotoxicity profile of CAR-T-cell therapy. The activation of CAR-T cells can disrupt the blood-brain and blood-ocular barriers, allowing infiltration of pro-inflammatory cytokines and CAR-T cells into the central nervous system, retina, and optic nerve. This inflammatory cascade may underlie both optic nerve damage and other ocular manifestations. As of now, no standardized treatment strategy for CAR-T-associated optic neuropathy has been established.
Beyond immune-mediated effects, reactivation of latent infections represents another important concern following CD19-directed CAR-T-cell therapy. The profound and often prolonged cytopenia that can occur—frequently as a result of prior chemotherapy or immunomodulatory treatments—can significantly weaken the immune system, leaving patients vulnerable to opportunistic infections. Among the reported ocular complications are cytomegalovirus (CMV) retinitis and varicella zoster virus (VZV) retinitis, both of which can pose serious threats to vision. Fortunately, when identified early, these infections have generally responded well to antiviral therapy, such as ganciclovir.
With the expanding application of CAR-T-cell therapy and its growing patient population, close ophthalmologic and infectious disease surveillance is critical. Any patient presenting with visual disturbances or experiencing delayed hematologic recovery should be carefully evaluated. Early recognition and prompt intervention can help prevent irreversible vision loss and improve overall patient outcomes.
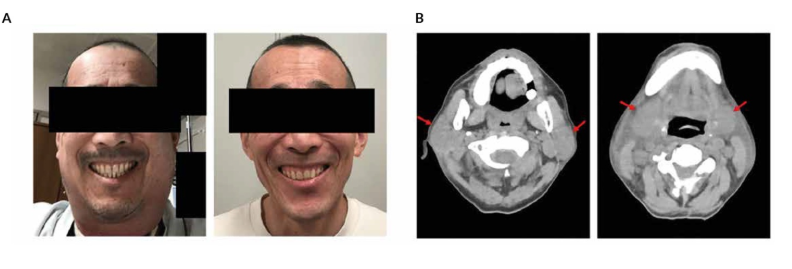
Cytokine release syndrome (CRS)-induced cervical edema. A) Gross appearance of cervical swelling at the emergence of local CRS on Day 5 post-chimeric antigen receptor (CAR) T-cell infusion (left) and after CRS resolution (right). B) Computed tomography (CT) scan of the neck area on Day 6 post-CAR T-cell infusion showing swelling of bilateral parotid glands (left, red arrows) and submaxillary glands (right, red arrows).
How Should We Diagnose Ocular Side Effects Of CAR-T Cell Therapy ?
The evaluation of suspected ocular toxicity following CAR T-cell therapy requires a multimodal diagnostic approach. Ophthalmologic assessment is the first step: fundoscopy is essential to detect optic nerve involvement such as papilledema and retinal abnormalities like detachment; pupillary reflex testing can reveal impaired light responses associated with neurotoxicity; and visual acuity assessment allows for monitoring of functional visual changes over time. Imaging plays a crucial role—ocular ultrasound or orbital MRI can identify intraocular complications, including anterior chamber infiltration or retinal detachment, while brain MRI helps exclude central nervous system (CNS) involvement such as edema or ischemia that may secondarily affect vision.
Laboratory and biomarker analysis can provide supportive evidence: elevated cytokines (e.g., IL-6, IL-8, MCP-1) in cerebrospinal fluid may reflect inflammatory neuro-ocular toxicity, and markers of blood-brain barrier disruption such as neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) in serum or CSF can indicate the extent of neuronal injury. Finally, a careful differential diagnosis is necessary. CNS infections must be ruled out, particularly in immunosuppressed patients, using lumbar puncture when safe. Disease relapse, including intraocular leukemic infiltration, should also be considered and may require targeted imaging or biopsy for confirmation.
How Should Ocular Side Effects from CAR T-Cell Therapy Be Managed?
Ocular side effects from CAR T-cell therapy require early recognition and a comprehensive management approach involving supportive care and targeted treatment of both systemic and local complications. Regular ophthalmic monitoring is essential for early detection of visual disturbances, optic neuropathy, retinal detachment, or ocular relapse of malignancy. Systemic toxicities such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) may contribute to ocular symptoms and should be managed promptly with supportive care, corticosteroids, and IL-6 receptor antagonists like tocilizumab.
Local ocular manifestations, including retinal infiltration, detachment, or opportunistic infections such as cytomegalovirus retinitis, may require specific therapies such as intravitreal injections (e.g., methotrexate, antivirals), corticosteroids, or surgical intervention. Before using this medication, please consult your doctor. Due to the immunosuppressive effects of lymphodepletion and CAR T-cell therapy, vigilant monitoring for opportunistic infections is critical.
Before initiating any treatment, it is essential to consult both your oncologist and ophthalmologist to ensure safe and appropriate management, minimizing potential risks to your health.
You Can Also Read Ocular Side Effects Of Immune Checkpoint Inhibitors by Oncodaily

How Can Oncologic and Ophthalmologic Expertise Be Integrated in Managing Immunotherapy Side Effects?
A multidisciplinary approach involving oncologists, ophthalmologists, and other relevant specialists is essential to ensure comprehensive care in patients undergoing CAR T-cell or other immunotherapies associated with ocular toxicities. Close collaboration enables the timely identification, evaluation, and management of ocular side effects while maintaining the overall efficacy of cancer treatment. This team-based strategy is particularly crucial in cases of moderate to severe ocular involvement, where treatment decisions—such as temporary discontinuation, dose modification, or initiation of targeted ocular therapy—must carefully weigh the risks of vision loss against the benefits of continued oncologic control. Regular communication between specialties supports individualized patient care and optimizes both oncologic and visual outcomes.
You Can Also Read Dr. Jason Luke’s Journey in Immuno-Oncology Innovation by OncoDaily
Written by Margarita Pozlikyan MD
FAQ
What ocular side effects can occur with CAR T-cell therapy?
Reported ocular effects include blurred vision, vision impairment, dry eyes, mydriasis (dilated pupils), pupillary abnormalities, conjunctivitis, optic neuritis, papilledema, retinal detachment, and infections like herpes zoster ophthalmicus and cytomegalovirus retinitis.
How common are ocular side effects in CAR T-cell therapy patients?
Ocular adverse events are relatively rare, occurring in about 1.6% to 1.9% of patients, with symptoms like vision changes, inflammation, and dry eyes being the most prevalent.
Are ocular side effects directly caused by CAR T-cells or secondary to other complications?
Many ocular issues arise secondary to systemic effects such as cytokine release syndrome (CRS), immune suppression, or inflammation triggered by CAR T-cell therapy rather than direct ocular toxicity.
What is the relationship between cytokine release syndrome and ocular symptoms?
CRS can cause systemic inflammation that may lead to ocular manifestations like exudative retinal detachment and papilledema due to increased cytokine levels and immune activation.
Are ocular toxicities included in CAR T-cell therapy product information?
Some ocular adverse events, including pupillary abnormalities and eye surface bleeding, have been detected in post-marketing data but are not yet documented in official product labels, highlighting the need for vigilance.
What is the recommended monitoring or management for ocular side effects?
Routine eye screening is not generally required for patients without pre-existing eye conditions, but symptomatic patients should receive prompt ophthalmologic evaluation and early intervention to prevent severe outcomes.
Can ocular side effects be severe or life-threatening?
While most ocular side effects are mild or moderate, some, such as retinal necrosis or optic neuritis, can be serious and may require urgent treatment to avoid permanent vision loss.
What is CAR T-cell therapy and how does it work?
CAR T-cell therapy is a type of immunotherapy that uses genetically modified T cells from a patient’s own immune system to recognize and kill cancer cells. The T cells are collected, engineered in a lab to express chimeric antigen receptors (CARs) that target specific proteins on cancer cells, expanded, and then infused back into the patient to attack the cancer.
Which types of cancer can CAR T-cell therapy treat?
CAR T-cell therapy is primarily used for certain blood cancers, including acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, mantle cell lymphoma, multiple myeloma, and other B-cell malignancies. The FDA has approved CAR T therapies for these cancers, especially when they are relapsed or refractory to prior treatments.
What are the common side effects and risks of CAR T-cell therapy?
Common side effects include cytokine release syndrome (CRS), which causes fever, fatigue, and low blood pressure; immune effector cell-associated neurotoxicity syndrome (ICANS), which can cause confusion and seizures; and other risks such as infections due to immune suppression. Patients require close monitoring after treatment due to these potentially severe side effects.