The METROPHOLYS trial (2692MO), presented by Dr. Antonella Brunello at the ESMO 2025 Congress, was a multicenter, open-label Phase II study designed to evaluate chemotherapy options for older adults with metastatic soft tissue sarcoma (STS). Conducted across leading European sarcoma centers, the trial sought to fill a critical evidence gap: while most pivotal STS trials have shaped standards of care, they have historically excluded or underrepresented patients aged 70 years and older.
As a result, oncologists often face uncertainty when balancing efficacy and tolerability in this population, who frequently present with comorbidities, reduced physiological reserve, and distinct treatment priorities. METROPHOLYS aimed to provide much-needed prospective data to better inform real-world therapeutic decisions for this growing demographic.
Background
Soft tissue sarcoma (STS) incidence rises steadily with age, yet older adults remain underrepresented in clinical trials, leading to uncertainty about the optimal management approach in this population. Treatment guidelines are often based on studies conducted in younger, fitter patients who can better tolerate intensive regimens. Doxorubicin (DOXO) continues to be the standard first-line therapy for metastatic STS, offering established efficacy but limited by its cardiotoxicity, myelosuppression, and cumulative dose constraints. These toxicities frequently pose challenges in elderly or frail patients, resulting in early discontinuation or the need for less intensive alternatives.
Metronomic cyclophosphamide (MC)—administered orally at low, continuous doses—has emerged as a potentially safer and more convenient option for this subgroup. By maintaining steady drug exposure, MC exerts antiangiogenic and immunomodulatory effects while minimizing severe hematologic and cardiac toxicity. This approach may allow older patients to sustain treatment longer, preserve quality of life, and achieve meaningful disease control in settings where standard-dose chemotherapy is not well tolerated.

Methods
Eligible patients (ECOG 0–2; age ≥ 70 years) were randomized 1:1 to:
- DOXO arm: 60 mg/m² every 3 weeks for up to 6 cycles
- MC arm: 50 mg daily orally until progression or intolerance
- Randomization was stratified by age (≤ 75 vs > 75 years), performance status, and G8 geriatric score (≤ 14 vs > 14).
The primary endpoint was time to treatment failure (TTF), defined as the interval from randomization to treatment discontinuation for progression, toxicity, or death.Secondary endpoints included progression-free survival (PFS), overall survival (OS), objective response rate (ORR), and safety.
Results of METROPHOLYS Trial
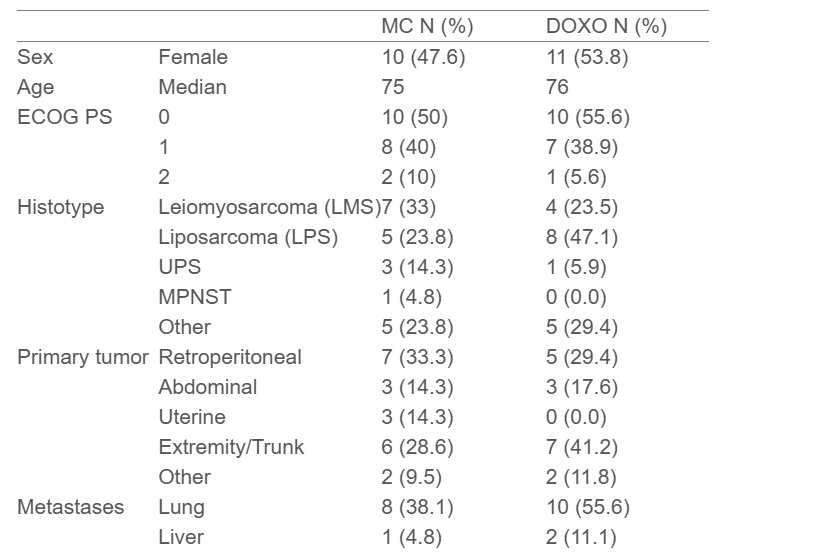
Between 2018 and 2023, 42 patients were enrolled (21 MC, 18 DOXO) before premature closure for accrual futility. Median age was 75–76 years, and leiomyosarcoma and liposarcoma were the most frequent histologies.
- Among 39 treated patients, any-grade adverse events occurred in 66.7% (MC) and 88.9% (DOXO); serious adverse events were reported in 8 patients (4 per arm).
- Median TTF was 2.3 months for MC and not reached for DOXO, with no statistically significant difference.
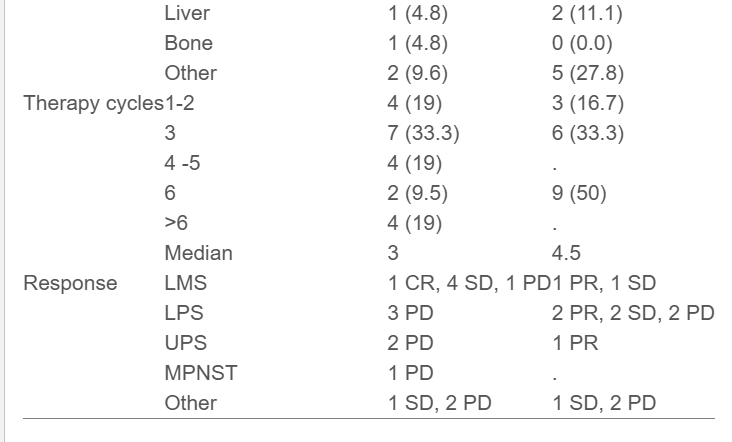
- ORR favored DOXO (30.8%) versus MC (6.7%), mainly driven by partial responses in liposarcoma and undifferentiated pleomorphic sarcoma subtypes.
- Median PFS was 4.1 months (DOXO) and 2.3 months (MC); OS was 19.1 months and 20.9 months, respectively—differences that were not statistically significant.
- Baseline G8 score ≤ 14 was observed in 68% of patients and did not correlate with outcome measures.
Key Insights
This study provides valuable prospective evidence on chemotherapy tolerance and activity in older patients with metastatic STS. Both regimens were feasible, and overall survival was longer than expected based on retrospective data, reflecting improved multidisciplinary and supportive care.
While DOXO achieved higher response rates, TTF, PFS, and OS did not differ significantly between arms, suggesting that metronomic cyclophosphamide may remain a reasonable option for frail or ineligible patients.
Clinical Significance
This trial highlights the critical need for age-adapted clinical research in soft tissue sarcoma, a field where evidence for elderly patients remains limited.
This study offers important prospective data on the efficacy and safety of chemotherapy in older patients with soft tissue sarcoma (STS). Despite multicenter collaboration, it was terminated early due to slow accrual. Although the limited sample size prevents definitive statistical comparisons, the observed TTF, PFS, OS, and toxicity profiles were similar between DOXO and MC, with overall survival outcomes exceeding those reported in previous retrospective studies.
You can read the full abstract here.


