Invasive lobular carcinoma (ILC), the second most common type of breast cancer, accounts for 10-15% of cases and is characterized by distinct histopathological and clinical features compared to invasive ductal carcinoma (IDC). Traditional prognostic tools often fail to capture these differences, potentially leading to suboptimal treatment decisions. The MammaPrint 70-gene signature assay has shown promise in risk stratification for early-stage breast cancer, but its applicability to ILC remains underexplored. This study, an exploratory subgroup analysis within the MINDACT trial, evaluates the prognostic value of the 70-gene signature in ILC, aiming to refine risk assessment and improve personalized treatment strategies.
Title: Survival outcomes for patients with invasive lobular cancer by MammaPrint: Results from the MINDACT phase III trial
Authors
Metzger Filho, F. Cardosob, C. Poncet, C. Desmedt, S. Linn, J. Wesseling, F. Hilbers, S. Delaloge, J.-Y. Pierga, E. Brain, S. Vrijaldenhoven, P.A. Neijenhuis, E.J.Th Rutgers, M. Piccart, L.J. van ’t Veer, G. Viale
Published in EJC, march 2025
Background
Invasive lobular carcinoma (ILC) represents approximately 10-15% of all invasive breast cancers, distinguishing itself from the more prevalent invasive ductal carcinoma (IDC) by unique histological and clinical characteristics.
Traditional prognostic tools often fail to account for these distinctions, leading to potential underestimation of recurrence risks in ILC patients. The MammaPrint 70-gene signature assay has demonstrated prognostic value in early-stage breast cancer, but its specific applicability to ILC has remained underexplored. This gap underscores the necessity for tailored prognostic assessments to inform adjuvant treatment decisions effectively.
Study Design of MINDACT trial
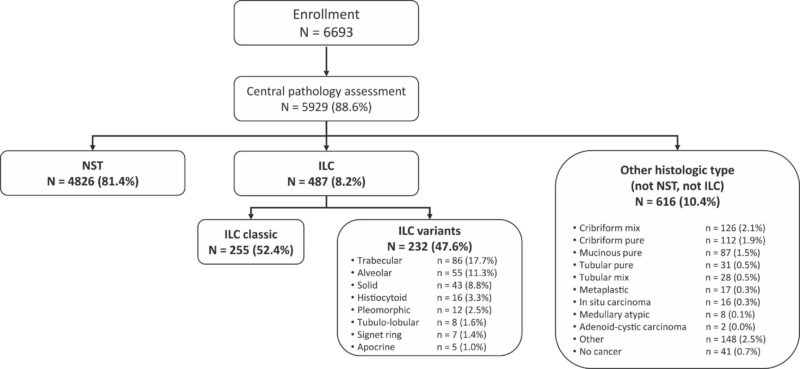
The study presents an exploratory subgroup analysis within the context of the MINDACT (Microarray In Node-negative and 1 to 3 positive lymph node Disease may Avoid ChemoTherapy) phase III trial. The primary objective was to evaluate the prognostic performance and clinical utility of the MammaPrint 70-gene signature in patients diagnosed with ILC. The analysis encompassed 5,929 patients with centrally assessed histology, categorized as either ILC or invasive breast cancer of no special type (NST). Patients were stratified based on genomic risk, as determined by the 70-gene signature, and clinical risk, assessed via a modified version of Adjuvant!Online.
Patient Population
Out of the total cohort, 487 patients were identified with ILC, and 4,826 with NST. The ILC group was further subclassified into classic ILC (255 patients) and ILC variants (232 patients). This stratification aimed to discern potential prognostic differences within ILC subtypes.
Results of MINDACT trial
Genomic Risk Classification
The MammaPrint 70-gene signature classified 16.2% of ILC patients and 39.1% of NST patients as genomic high-risk (gH). Within the ILC cohort, 10.2% of classic ILC and 22.8% of ILC variants were designated as gH.
Survival Outcomes
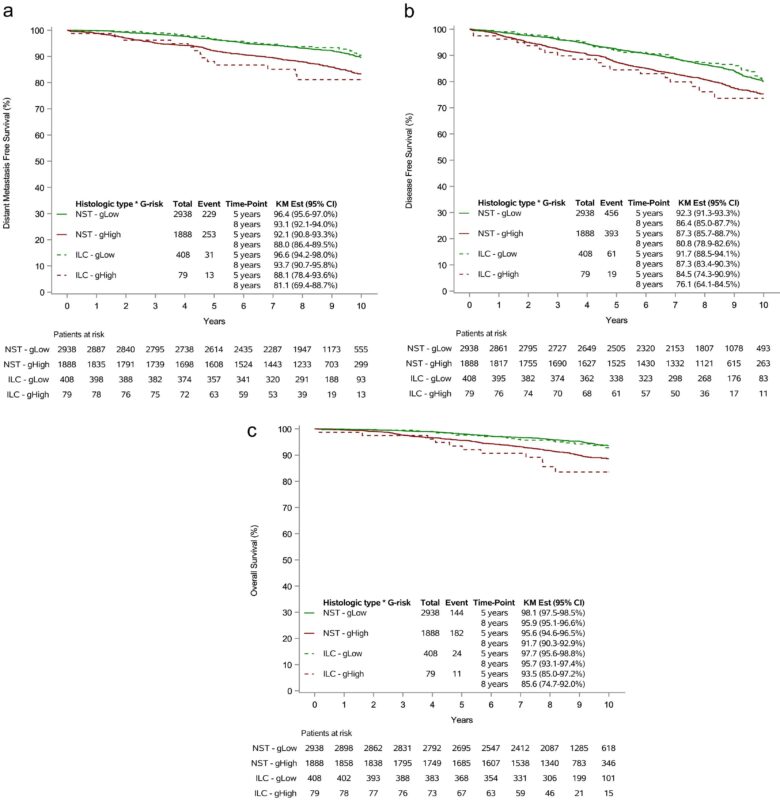
At a median follow-up of 8.7 years, survival outcomes were analyzed across genomic risk groups:
- Disease-Free Survival (DFS): The 5-year DFS estimate for ILC variants was 88.4% (95% CI: 83.1–92.1), compared to 93.0% (95% CI: 88.7–95.7) for classic ILC.
- Comparative Survival: Survival estimates for ILC and NST patients were similar when stratified by genomic risk, indicating the 70-gene signature’s consistent prognostic value across these histological types.
Key Findings
The findings from this analysis underscore the prognostic significance of the MammaPrint 70-gene signature in invasive lobular carcinoma. By effectively stratifying patients based on genomic risk, this assay facilitates more personalized and informed adjuvant treatment decisions. The study highlights the importance of considering histological subtypes and integrating genomic tools to optimize outcomes for ILC patients.
- Prognostic Stratification: The 70-gene signature effectively identified a subset of ILC patients (16.2%) with high genomic risk, correlating with unfavorable survival outcomes.
- Subtype Variations: ILC variants exhibited a higher proportion of high genomic risk classification (22.8%) compared to classic ILC (10.2%), suggesting intrinsic biological differences that may influence prognosis.
- Chemotherapy Considerations: The study suggests that omitting chemotherapy could be considered for ILC patients with high clinical risk but low genomic risk, as determined by the 70-gene signature.
Key Takeaway Messages
- Clinical Utility of MammaPrint: The 70-gene signature serves as a valuable prognostic tool in ILC, aiding in the identification of patients who may benefit from adjuvant chemotherapy, thereby personalizing treatment strategies.
- Histological Considerations: Recognizing the heterogeneity within ILC subtypes is crucial, as it impacts genomic risk classification and subsequent management decisions.
- Treatment Optimization: Integrating genomic risk assessment with traditional clinical factors can refine adjuvant treatment recommendations, potentially sparing patients from unnecessary chemotherapy and its associated toxicities.
You can read the full article here
Written by Sona Karamyan, MD


