Immune checkpoint inhibitors targeting the PD-1 pathway have transformed outcomes in recurrent or metastatic head and neck squamous cell carcinoma (HNSCC).
Pivotal international trials such as CheckMate-141 and KEYNOTE-048 established nivolumab and pembrolizumab as survival-improving standards using conventional systemic dosing.
Despite these advances, global implementation remains profoundly unequal.
In many low- and middle-income countries (LMICs), including India, only 1–3% of eligible patients can access full-dose immunotherapy because of cost constraints.
This disparity prompted Indian investigators to explore a biologically and clinically provocative hypothesis:
Could substantially lower doses of PD-1 blockade preserve antitumor efficacy while expanding real-world access?
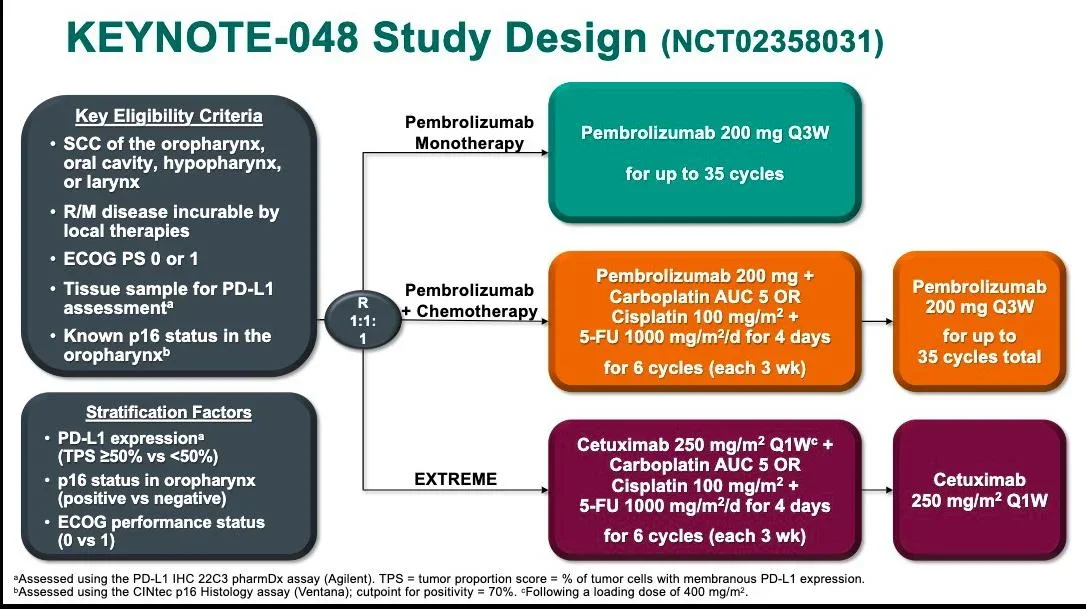
Standard-Dose Immunotherapy: Evidence From Global Trials
CheckMate-141 (nivolumab vs investigator’s choice)
- Population: platinum-refractory recurrent/metastatic HNSCC
- Total patients randomized: 361
- Dose: nivolumab 3 mg/kg every 2 weeks
- Median overall survival (OS): ~7.5 months vs ~5.1 months
- Hazard ratio for death: ~0.70
This trial defined nivolumab as a global standard in the second-line setting.
KEYNOTE-048 (pembrolizumab-based first-line therapy)
- Established pembrolizumab monotherapy or chemo-immunotherapy
as first-line standard in PD-L1–selected disease.
Together, these studies anchored modern HNSCC immunotherapy around full-dose systemic exposure.
The Indian Strategy: Testing Ultra-Low-Dose PD-1 Blockade
A landmark phase III randomized study led by Patil et al. and published in the Journal of Clinical Oncology (2023) explored whether substantially reduced PD-1 inhibitor dosing could still deliver meaningful clinical benefit in head and neck cancer.
In this trial, patients received triple metronomic chemotherapy (TMC)—a low-cost oral regimen consisting of methotrexate, celecoxib, and erlotinib—either alone or in combination with ultra-low-dose nivolumab administered at 20 mg every three weeks, representing only ~6–10% of conventional dose intensity.
Clinical outcomes
The addition of low-dose nivolumab resulted in clear and clinically relevant survival improvements:
- Total patients randomized: 151
- One-year overall survival:
43.4% vs 16.3% - Median overall survival:
10.1 vs 6.7 months - Median progression-free survival:
6.6 vs 4.6 months
Importantly, the rate of grade ≥ 3 toxicities remained comparable between treatment arms, indicating that the survival benefit was achieved without an increase in severe adverse events.
Interpretation
Together, these findings demonstrate that meaningful survival benefit can be achieved with dramatically reduced nivolumab exposure, challenging traditional assumptions that effective checkpoint inhibition requires full systemic dosing.
Induction chemo-immunotherapy in locally advanced HNSCC
Cohort analyses of stage III–IVB disease treated with low-dose nivolumab–based induction therapy reported:
- Overall response rate: ~75%
- Conversion to resectability: ~32%
- Pathologic complete response: ~32%
- 1-year OS: ~83%
Drug expenditure decreased by ≈90% vs standard dosing, without excess severe toxicity.
Interpretation: What Do These Trials Prove – And What Do They Not?
Supported conclusions
- Ultra-low-dose nivolumab shows clear clinical activity.
- Survival improves vs non-immunotherapy regimens.
- Toxicity remains acceptable or reduced.
- Cost reductions approach 90%, dramatically expanding potential access.
Remaining uncertainties
- No direct randomized comparison of low- vs standard-dose nivolumab exists.
- Cross-trial comparisons with CheckMate-141 or KEYNOTE-048 cannot establish non-inferiority or superiority.
- Generalizability beyond HNSCC and LMIC settings remains unclear.
Conclusion
The Indian phase III trial of ultra-low-dose nivolumab represents one of the most thought-provoking contemporary contributions to immuno-oncology.
By demonstrating clinically meaningful survival improvement without increased toxicity at only a fraction of conventional PD-1 inhibitor dosing, this study challenges the long-standing assumption that effective immunotherapy requires maximal systemic exposure.
Importantly, these findings extend beyond a single regimen or tumor type.
They introduce a broader conceptual shift—from dose intensity toward biologic sufficiency, and from drug innovation alone toward innovation in access.
However, the absence of direct randomized comparisons between low- and standard-dose PD-1 blockade means that non-inferiority or superiority cannot yet be concluded.
Future global trials integrating dose optimization, pharmacodynamic biomarkers, and health-economic endpointswill be essential to determine whether reduced-dose immunotherapy can safely redefine international treatment standards.
Until such data emerge, the Indian experience stands as a powerful reminder that progress in oncology is measured not only by discovering new therapies, but by ensuring that effective treatment becomes realistically accessible to patients worldwide.

You Can Also Read Head and Neck Cancer: Hidden Risks, Mutations and Outcomes