Immunotherapy is no longer just for advanced lung cancer—it’s moving into the curative arena. Across ongoing trials, researchers are pairing checkpoint inhibitors with chemotherapy, antibody–drug conjugates, radiotherapy, and even cryoablation to attack tumors before and after surgery. The strategy is bold: shrink cancers early, wipe out hidden micrometastases, and carry immune momentum through recovery.
From perioperative regimens like sintilimab–bevacizumab–chemo to novel concepts such as toripalimab with cryoablation or spatially fractionated radiotherapy, these approaches aim to redefine what is possible. The ultimate goal is not just extending survival—but achieving true long-term remission, and perhaps cure, in lung cancer.
Preoperative Sintilimab + Bevacizumab + Chemotherapy in Resectable NSCLC
A single-arm clinical trial is investigating a novel neoadjuvant regimen for patients with resectable stage II–IIIA non-small cell lung cancer (NSCLC). The study combines:
-
Sintilimab (a PD-1 checkpoint inhibitor),
-
Bevacizumab (an anti-VEGF antibody), and
-
Platinum-based chemotherapy (carboplatin + pemetrexed),
all given before surgery.
In this open-label study, patients receive:
-
Sintilimab 200 mg IV every 3 weeks × 4 cycles
-
Bevacizumab 15 mg/kg IV every 3 weeks × 4 cycles
-
Carboplatin (AUC=5) + pemetrexed (500 mg/m²) IV every 3 weeks × 4 cycles
Following treatment, patients undergo surgical resection within 4–6 weeks. Mid-treatment CT scans assess radiographic response, and patients with progressive disease may proceed to earlier surgery.
The primary goal is to evaluate safety and tolerability of the combination, measured by the rate of severe treatment-related adverse events. Secondary objectives include feasibility (completion of therapy and surgery), radiographic response, and major pathological response (≤10% residual viable tumor at surgery).
The trial aims to enroll 20 patients, with completion expected in December 2025. If feasible and safe, this approach could lay the groundwork for larger studies testing whether dual immunotherapy and anti-angiogenic therapy can enhance neoadjuvant outcomes in resectable NSCLC.
The Real-World Study of Neoadjuvant Immunotherapy in Early-Stage NSCLC
A prospective, real-world study is evaluating the effectiveness of neoadjuvant immunotherapy in patients with resectable stage II–IIIA non-small cell lung cancer (NSCLC). The study reflects real clinical practice in China, assessing both single-agent PD-1/PD-L1 inhibitors and immunotherapy combined with chemotherapy before surgery.
Patients included must have EGFR- and ALK-negative tumors, good performance status, and resectable disease. Treatments are delivered according to physician choice (monotherapy or chemo-immunotherapy), followed by surgical resection.
The primary aim is to determine the rate of major pathological response (MPR) after neoadjuvant immunotherapy. Secondary endpoints include:
-
Objective response rate (ORR) by RECIST 1.1
-
Pathological complete response (pCR) rate
-
MPR across PD-L1 subgroups (positive vs negative expression)
-
Safety and tolerability (adverse events, surgical delays)
-
Progression-free survival (PFS) with long-term follow-up up to 100 months
The trial plans to recruit 100 patients and is ongoing, with completion expected in 2029. Biomarker analyses are also embedded to explore predictors of response and to move toward personalized neoadjuvant immunotherapy.
Spatially Fractionated + Low-dose Radiotherapy for Immunotherapy/Chemotherapy-Resistant NSCLC
A Phase II randomized trial in China is testing a novel radiotherapy approach to overcome resistance in patients with locally advanced inoperable or advanced NSCLC who have progressed after chemo-immunotherapy. The strategy uses spatially fractionated high-dose radiation combined with low-dose radiotherapy alongside systemic treatment.
Patients are randomized 1:1 into two groups.
-
Experimental arm: One lesion receives spatially fractionated radiotherapy (high-dose regions 800–1200 cGy × 3; low-dose regions 100–300 cGy × 5). All other irradiable metastases receive low-dose radiotherapy (100–300 cGy × 5). Immunotherapy plus chemotherapy is given during or within a week after radiotherapy.
-
Control arm: Patients receive standard fractionated radiotherapy to thoracic or other targets, followed by immunotherapy and chemotherapy based on disease stage, tolerance, and molecular profile.
The trial plans to enroll 84 patients. Eligibility requires prior immunotherapy exposure with progression, KPS ≥70, and resectable radiotherapy targets. Patients with severe comorbidities, autoimmune disease, or uncontrolled organ dysfunction are excluded.
The primary endpoint is objective response rate (ORR) at 6 months.
Secondary endpoints include disease control rate, progression-free survival, overall survival, and safety (graded by CTCAE v5.0 and RTOG). Blood and tissue samples are collected before and after treatment to analyze immune cell subsets and cytokines, offering insights into how radiation reshapes the tumor immune microenvironment.
The study began in October 2024 with expected primary completion in January 2027 and final completion in January 2028.
Chemo-immunotherapy Plus Thoracic Radiotherapy in ES-SCLC
The TRIPLEX phase III trial is testing whether adding thoracic radiotherapy improves survival for patients with extensive-stage small-cell lung cancer (ES-SCLC) treated with standard chemo-immunotherapy (carboplatin, etoposide, and durvalumab). Patients are randomized to receive chemo-immunotherapy alone or the same regimen plus 30 Gy in 10 fractions of thoracic radiotherapy between the second and third cycles, followed by durvalumab maintenance.
The primary endpoint is one-year overall survival, with secondary measures including progression-free survival, long-term survival, response rates, thoracic local control, safety, quality of life, and cognitive outcomes related to prophylactic cranial irradiation. Biological samples are being collected for biomarker studies to identify predictors of benefit.
With a target enrollment of 302 patients across several European countries, the study seeks to determine whether radiotherapy can act synergistically with PD-L1 blockade to extend the modest survival benefit currently achieved with chemo-immunotherapy in ES-SCLC.
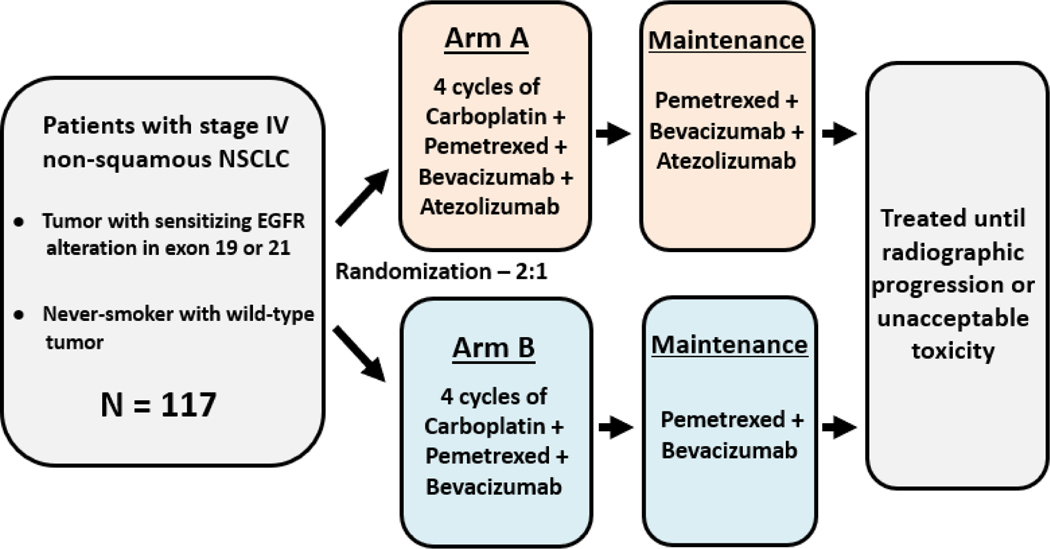
Carboplatin + Pemetrexed + Bevacizumab With or Without Atezolizumab in Stage IV NSCLC
The TH-138 phase II randomized trial is evaluating whether adding the PD-L1 inhibitor atezolizumab to standard carboplatin + pemetrexed + bevacizumab improves outcomes in stage IV non-squamous NSCLC patients who are either never smokers or have tumors harboring EGFR exon 19/21 mutations.
Patients are randomized to:
-
Arm A (experimental): carboplatin + pemetrexed + bevacizumab + atezolizumab for four cycles, followed by maintenance with pemetrexed, bevacizumab, and atezolizumab.
-
Arm B (control): the same triplet regimen without atezolizumab, with pemetrexed + bevacizumab as maintenance.
The primary endpoint is progression-free survival (PFS), with secondary outcomes including overall response rate (ORR), duration of response, and safety (CTCAE v5.0). A total of 117 patients are expected to be enrolled, with study completion projected in 2028.
This trial specifically addresses the subset of NSCLC patients who are never smokers or EGFR-mutated, aiming to determine whether combining immunotherapy with chemotherapy and anti-angiogenic therapy can improve survival beyond current standards.
Toripalimab Combined With Cryoablation for Oligo-progression in Advanced NSCLC
This single-arm phase II study is testing the PD-1 inhibitor toripalimab together with cryoablation in patients with driver-negative advanced NSCLC who develop limited (oligometastatic) progression after first-line immunotherapy. Cryoablation is used to locally destroy 1–5 metastatic lesions in up to three organs, with the aim of enhancing systemic immune response. Toripalimab (240 mg IV every 3 weeks) begins on day 3 after cryoablation and continues until progression or unacceptable toxicity.
The primary endpoint is progression-free survival (PFS) at 6 months. Secondary outcomes include objective response rate (ORR), disease control rate (DCR), overall survival (OS up to 24 months), safety, and correlation of PD-L1 expression with response. The trial plans to enroll 54 patients in China, with completion expected in 2028.
Neoadjuvant Sugemalimab + Chemotherapy Followed by Adjuvant Sugemalimab in Resectable Stage II–IIIA NSCLC
This single-arm, phase II exploratory trial is testing perioperative sugemalimab in patients with resectable stage II–IIIA NSCLC without EGFR or ALK mutations. The study aims to evaluate feasibility, safety, and efficacy, while also exploring immune microenvironment changes. A total of 25 patients will be enrolled, with at least 40% having squamous histology.
Patients receive 3–4 cycles of neoadjuvant sugemalimab (1200 mg IV every 3 weeks) plus platinum chemotherapy, followed by surgery. Those with complete resection continue on adjuvant sugemalimab every 3 weeks for 1 year, with optional adjuvant chemotherapy as appropriate. Tumor tissue is collected at baseline and after surgery for genomic and immune profiling (multiplex immunofluorescence, single-cell sequencing, RNA-seq, WES).
The primary endpoint is major pathological response (MPR), defined as ≤10% viable tumor cells at surgery. Secondary measures include pathologic complete response (pCR), event-free survival (6–18 months), safety, and translational analyses of the immune microenvironment. Recruitment began in June 2025, with completion expected by 2028.
Post-operative Radiotherapy After Neoadjuvant Chemo-immunotherapy and Surgery in Stage III NSCLC
This phase II randomized trial is evaluating whether post-operative radiotherapy (PORT) improves outcomes in patients with stage III NSCLC who have residual nodal disease after neoadjuvant chemo-immunotherapy and complete surgical resection. The study will enroll 80 patients and stratify by use of adjuvant immunotherapy.
Participants are randomized to either mediastinal PORT (40 Gy in 15 fractions within 24 weeks after surgery) or observation without PORT. The primary endpoint is event-free survival (EFS), with a goal of detecting a 15% improvement in 3-year EFS. Secondary endpoints include overall survival, locoregional control, distant metastasis-free survival, and grade 3 toxicity, with follow-up extending up to 5 years.
Recruitment is expected to begin in May 2025, with primary completion in 2028 and full study completion in 2030.
Neoadjuvant Sacituzumab Govitecan Plus Pembrolizumab in Resectable NSCLC
This phase II, open-label, single-arm multicenter study is evaluating the combination of sacituzumab govitecan (a TROP-2–directed antibody–drug conjugate) and pembrolizumab in EGFR/ALK-negative stage II–III resectable NSCLC. Patients receive 4 cycles of neoadjuvant pembrolizumab + sacituzumab govitecan, followed by surgery, and then 13 cycles of maintenance pembrolizumab.
The primary endpoint is pathologic complete response (pCR) in the intention-to-treat population. Secondary endpoints include resection rate, major pathologic response (MPR), event-free survival (12- and 24-month rates), overall survival, objective response rate (ORR), safety (CTCAE v5.0), surgical outcomes, and patient-reported quality of life (EQ-5D-3L).
Treatment Plan:
-
Neoadjuvant: Pembrolizumab 200 mg IV (Day 1, Q3W) + Sacituzumab Govitecan 10 mg/kg IV (Day 1 & 8, Q3W) × 4 cycles.
-
Surgery: Within 4–8 weeks of last neoadjuvant cycle (post-op RT if R1/R2 resection).
-
Adjuvant: Pembrolizumab 200 mg IV Q3W × 13 cycles.
Eligibility: Adults ≥18 with resectable stage II–III NSCLC (AJCC 8th), EGFR/ALK negative, ECOG 0–1, adequate organ function, and available tumor tissue. Key exclusions include distant metastasis, prior PD-1/PD-L1 or TROP-2 therapy, chest radiotherapy, active autoimmune disease, chronic immunosuppression, uncontrolled cardiac/pulmonary disease, and active HBV/HCV/HIV infection.
Perioperative Tislelizumab for Resectable Small Cell Lung Cancer
This phase II, single-arm study evaluates perioperative tislelizumab in combination with platinum-etoposide chemotherapy for patients with resectable stage IIB–IIIB limited-stage SCLC. The regimen includes neoadjuvant tislelizumab + cisplatin/carboplatin + etoposide (2–4 cycles), followed by radical surgery for eligible patients, and then adjuvant tislelizumab ± chemotherapy, with tislelizumab maintenance for 1 year.
The primary endpoint is 1-year event-free survival (EFS). Secondary endpoints include long-term EFS, overall survival, objective response rate, disease control rate, pathological complete response (pCR), major pathological response (MPR), downstaging rates, surgical resection and R0 resection rates, and safety (treatment-emergent and immune-related adverse events).
Treatment Plan:
-
Neoadjuvant: Tislelizumab 200 mg IV Q3W + Cisplatin (75 mg/m² d1) or Carboplatin (AUC 5–6 d1) + Etoposide (100 mg/m² d1–3), 2–4 cycles.
-
Surgery: Radical resection for eligible patients.
-
Adjuvant: Completion of 4 total perioperative chemotherapy cycles with tislelizumab + platinum-etoposide, followed by tislelizumab monotherapy for 1 year.
Eligibility: Adults 18–75 with histologically confirmed limited-stage SCLC (IIB–IIIB, AJCC 8th) deemed resectable. ECOG 0–1, adequate organ function, and no prior systemic therapy. Exclusions include NSCLC histology, brain metastases, interstitial lung disease, uncontrolled cardiovascular disease, autoimmune disorders, active infections, pregnancy, or other recent malignancies.

You Can Also Read About 10 Ongoing Clinical Trials on Immunotherapy in Melanoma