Immunotherapy has become a major breakthrough in cancer treatment, helping the body’s own immune system recognize and fight cancer cells. Unlike traditional therapies, immunotherapy can have lasting effects, even after treatment ends. Understanding how long immunotherapy stays active in the body is important for managing side effects, planning future treatments, and monitoring patient outcomes.
This article explores the duration of immunotherapy’s presence and effects, the factors that influence it, and what patients and clinicians need to know about its lasting impact.
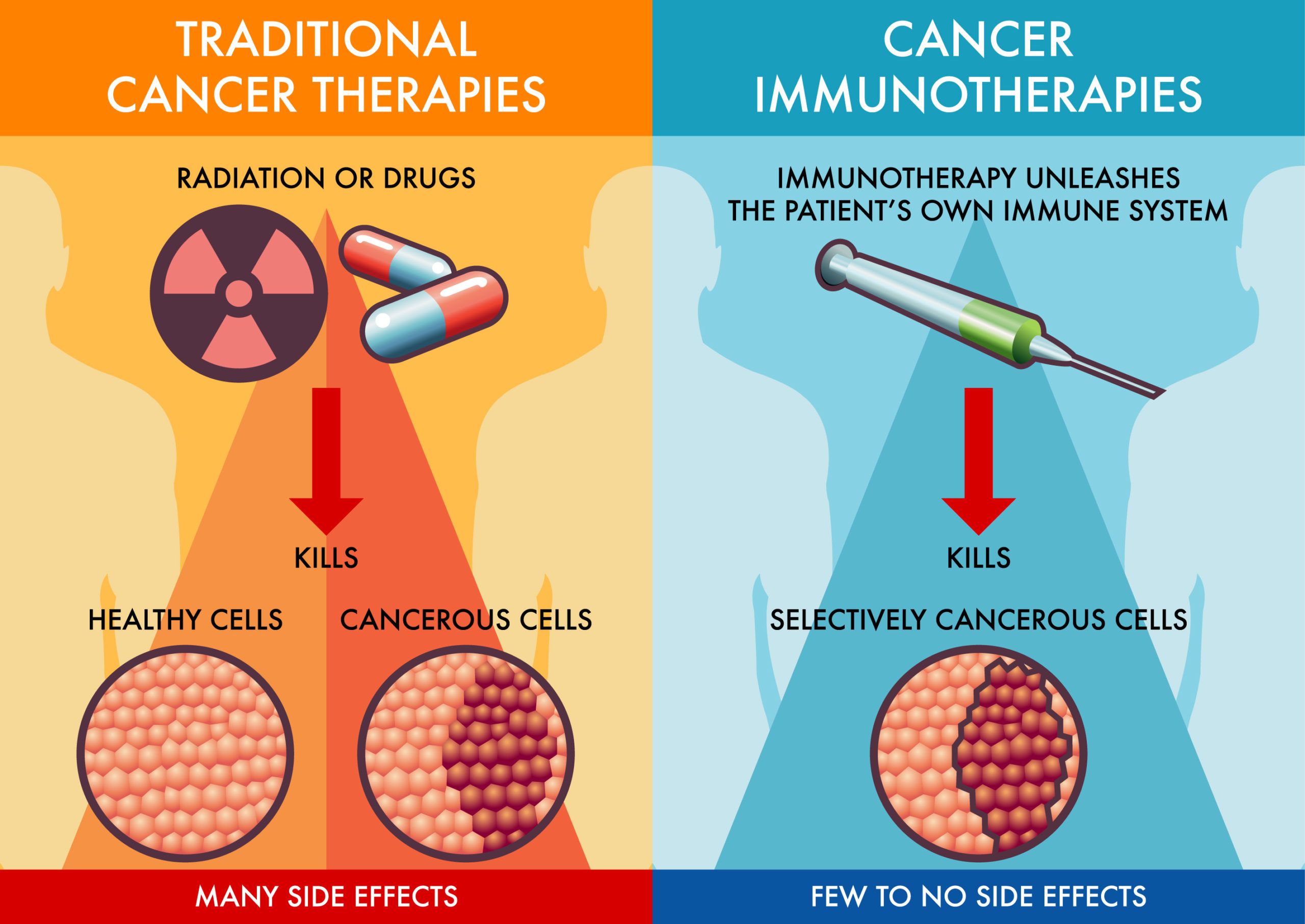
What Is Immunotherapy and How Does It Work?
According to the American Cancer Society, immunotherapy is a form of cancer treatment that utilizes a person’s own immune system to recognize and destroy cancer cells more effectively. This approach can enhance the immune response by targeting specific proteins involved in immune system regulation.
One common type of immunotherapy involves the use of immune checkpoint inhibitors. These drugs work by blocking checkpoint proteins, which are natural parts of the immune system that can sometimes prevent immune cells from attacking cancer cells. By inhibiting these checkpoints, the drugs enable a more robust immune response against the tumor. Examples of immune checkpoint inhibitors include:
-
Pembrolizumab (Keytruda): Targets the PD-1 protein on T cells, enhancing the immune system’s ability to detect and fight cancer cells.
-
Nivolumab (Opdivo): Also targets PD-1, and is used in the treatment of various cancers, including melanoma and lung cancer.
-
Atezolizumab (Tecentriq): Targets the PD-L1 protein, helping restore immune responses against cancer cells.
These therapies have been approved for the treatment of several cancer types and have shown significant efficacy in improving patient outcomes.
How Long Does Immunotherapy Stay in Your System?
According to the American Cancer Society, immunotherapy encompasses various treatments that harness the body’s immune system to combat cancer. The duration these drugs remain active in the body varies significantly based on their specific types and mechanisms.
Monoclonal antibodies, such as checkpoint inhibitors, are a prevalent form of immunotherapy. For instance, nivolumab (Opdivo), a PD-1 inhibitor, has a terminal half-life of approximately 26.7 days, reaching steady-state concentrations after 12 weeks of administration. Similarly, pembrolizumab (Keytruda), another PD-1 inhibitor, exhibits a terminal half-life of about 20 days. These extended half-lives allow for dosing intervals ranging from every 2 to 4 weeks. In contrast, cytokine-based therapies, like interleukin-2 (IL-2), have much shorter half-lives. IL-2 is rapidly cleared from the bloodstream, necessitating frequent dosing to maintain therapeutic levels.
Cell-based therapies, such as CAR-T cell treatments, involve administering modified immune cells that can persist and remain active in the body for extended periods. The longevity of these cells varies among individuals and is influenced by factors like the patient’s immune environment and disease characteristics. Understanding the pharmacokinetics of immunotherapy agents is crucial for optimizing treatment schedules and managing potential side effects. The persistence of these drugs in the system directly impacts their efficacy and the duration of immune-related adverse events.
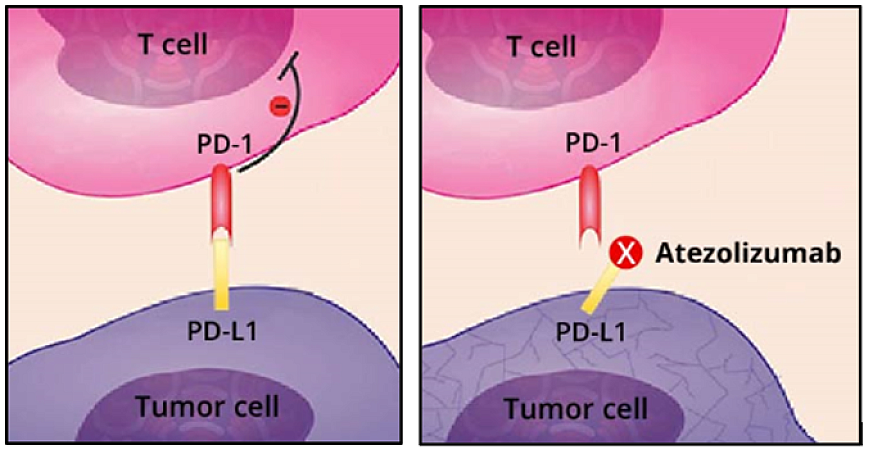
Factors Influencing Immunotherapy Duration
The amount of time immunotherapy stays in a patient’s system can vary widely, and it depends on several important factors. One of the most significant is the type of drug being used. The dosage and frequency of treatment also play a role. Higher doses or more frequent infusions can keep the drug active in the system for a longer period. However, some immunotherapy drugs are now given as fixed doses, simplifying treatment and making duration more predictable. A patient’s metabolism and overall health are also key. People with slower metabolism or reduced kidney and liver function may clear the drug more slowly, which means the effects could last longer. Similarly, those with a weakened immune system—due to age, chemotherapy, or chronic illness—might process the treatment differently.
Even body size can make a difference. Although many immunotherapies now use a one-size-fits-all approach, some are still given based on weight, which can influence how long the drug stays active. Finally, how the patient’s body responds to the cancer plays a major role. In people with a large tumor burden, the immune system may stay more activated for a longer time, extending the therapeutic effects. In cases where the immunotherapy triggers a strong immune response, those effects can persist well beyond the drug’s physical presence in the bloodstream.
Together, these factors explain why the timeline for immunotherapy’s presence and activity can be very different from person to person. Understanding them helps patients and doctors work together to monitor the treatment’s impact and manage any ongoing effects with greater precision.
How Long Does Allergy Immunotherapy Stay in Your System?
Allergy immunotherapy is a long-term treatment that helps reduce the immune system’s overreaction to allergens such as pollen, dust mites, or pet dander. There are two main forms: subcutaneous immunotherapy (SCIT), commonly known as allergy shots, and sublingual immunotherapy (SLIT), which involves taking drops or tablets under the tongue. Both approaches aim to gradually train the immune system to tolerate allergens, leading to long-lasting symptom relief even after treatment ends.
According to the American College of Allergy, Asthma & Immunology (ACAAI), SCIT typically lasts three to five years. The treatment begins with a build-up phase, where injections are given once or twice weekly, followed by a maintenance phase with injections spaced every two to four weeks. Similarly, SLIT is usually prescribed for at least three years, with daily self-administered doses taken at home after the first dose is given under medical supervision.
The question many patients have is: how long do the effects last after stopping treatment? Research shows that the benefits of allergy immunotherapy can persist well beyond the last dose—but duration matters. A study published in the New England Journal of Medicine, led by Durham et al., found that three years of grass-pollen SCIT led to sustained symptom relief lasting at least another three years after therapy was discontinued. In contrast, a more recent trial published in JAMA—the GRASS trial—showed that two years of immunotherapy (both SCIT and SLIT) was not enough to maintain long-term benefit after treatment ended (Scadding et al., JAMA, 2017).
These findings support current clinical guidelines recommending a minimum of three years of continuous allergy immunotherapy for lasting results. While responses can vary by patient, allergists use this timeline to help determine when it’s appropriate to stop treatment and whether additional years may be needed.
Monitoring Immunotherapy Effectiveness Over Time
Monitoring the effectiveness of immunotherapy is an essential part of cancer treatment, helping doctors assess whether the therapy is working, needs adjustment, or should be stopped. Because immunotherapy works differently than chemotherapy, its success is often measured using a combination of tools, including imaging tests, blood tests, and physical exams over time.
One of the most common methods is imaging, such as CT scans, MRI, or PET scans, which allow doctors to track changes in tumor size or appearance. For instance, a decrease in tumor size over several months may indicate that the immune system is successfully attacking cancer cells. However, with immunotherapy, doctors are also alert to a unique phenomenon called pseudoprogression—where tumors may appear larger at first due to immune cells infiltrating the tumor—before eventually shrinking. This highlights the importance of interpreting scans in the context of the patient’s overall condition. Blood tests are another key part of monitoring. These can measure tumor markers, inflammatory signals, or the presence of immune-related side effects. In some cases, doctors also assess circulating tumor DNA (ctDNA), which provides a non-invasive way to detect changes in tumor burden and identify early signs of resistance or recurrence.
Routine physical exams and symptom tracking are equally important. Doctors check for changes in weight, fatigue, and signs of immune-related adverse events (irAEs), such as rash, joint pain, or digestive issues, which may signal both treatment activity and toxicity. For example, a patient who develops mild colitis while on checkpoint inhibitors might be experiencing a side effect related to an activated immune system—but this can also indicate a strong therapeutic response. By combining these methods, clinicians can build a complete picture of how well immunotherapy is working and make informed decisions about whether to continue, pause, or change the treatment strategy. This approach ensures that patients receive the most effective care while minimizing risks.
Potential Long-Term Effects of Immunotherapy
Immunotherapy has significantly advanced cancer treatment by leveraging the body’s immune system to combat malignancies, resulting in sustained immune responses and prolonged remissions across various cancer types.
In advanced melanoma, the CheckMate 067 trial, led by Jedd D. Wolchok and published in Nature Cancer, reported 10-year survival outcomes for patients treated with a combination of nivolumab and ipilimumab. The study found that the median melanoma-specific survival exceeded 120 months for the combination therapy, with 37% of patients alive at the end of the trial. At the 10-year mark, melanoma-specific survival rates were 52% for the combination therapy, 44% for nivolumab monotherapy, and 23% for ipilimumab monotherapy.
In B-cell malignancies, a study by James N. Kochenderfer, published in the Journal of Clinical Oncology, demonstrated that anti-CD19 chimeric antigen receptor (CAR) T-cell therapy induced complete remissions lasting three years or more in 51% of evaluable treatments. Some remissions extended up to nine years, with late adverse events being rare.
Managing Side Effects After Immunotherapy
Immunotherapy has transformed cancer care, but like all treatments, it can cause side effects—some of which may continue even after the treatment has ended. These are called immune-related adverse events (irAEs), and they occur because immunotherapy activates the immune system, which can sometimes continue attacking healthy tissues even after cancer therapy stops.
One of the most common long-term side effects is fatigue. Many patients report lingering tiredness that doesn’t improve right away after treatment ends. To manage this, doctors may recommend gentle exercise, proper sleep routines, hydration, and sometimes referral to physical therapy or fatigue management programs. Joint pain and muscle aches (arthralgia and myalgia) are also frequently reported. These can be treated with nonsteroidal anti-inflammatory drugs (NSAIDs), stretching, or corticosteroids in more severe cases. Endocrine issues are another major concern. Checkpoint inhibitors can lead to long-term problems such as thyroid dysfunction, adrenal insufficiency, or type 1 diabetes. These conditions may require lifelong hormone replacement therapy, like levothyroxine or corticosteroids. Regular blood tests help monitor hormone levels and guide treatment.
Skin conditions, such as rashes or vitiligo, can also persist. Mild rashes may be treated with topical creams, while more extensive skin issues might require dermatologic care or oral medications.Some patients may also experience persistent digestive symptoms, like diarrhea or colitis, which stem from inflammation of the gut lining. Management includes dietary changes, hydration, and medications like corticosteroids or biologics in more serious cases. It’s important to know that these side effects vary from person to person. Regular follow-up appointments and communication with the oncology care team are key. Many cancer centers now have survivorship clinics to help manage long-term effects and improve quality of life after treatment.
While persistent side effects can be challenging, they are often manageable with the right care. Early detection and treatment can reduce their impact and help patients return to normal life more smoothly.
What Should Patients Discuss with Their Doctors?
Before starting or completing immunotherapy, it’s important for patients to have open, informed conversations with their healthcare team. Every person’s treatment is different, and understanding how long immunotherapy might stay in your system, what side effects to expect, and how to manage them can make a big difference in your care and peace of mind.
Patients should feel empowered to ask specific questions about how long the treatment will last, how their body will respond over time, and what happens after the treatment ends. This includes understanding both the short-term and long-term effects of the drugs and what kind of monitoring will be necessary once therapy concludes.A key topic to discuss is how success will be measured. Immunotherapy doesn’t always produce immediate results, and sometimes tumors may look larger on scans before they begin to shrink—a phenomenon known as pseudoprogression. Your doctor can explain what to expect during treatment and how progress will be tracked.
Another important area is side effect management. Patients should ask about what side effects could linger after treatment, how they can be treated, and what signs might indicate a need for immediate attention.Finally, discussing life after immunotherapy—including follow-up care, hormone monitoring (if applicable), and survivorship support—can help patients prepare for the next phase of their journey.
Questions to Discuss With Your Healthcare Provider
-
How long will I be receiving immunotherapy, and how often?
-
How long will the effects of immunotherapy last in my body?
-
What are the most common short-term and long-term side effects?
-
What symptoms should I report right away, even after treatment ends?
-
How will we monitor my progress (e.g., blood tests, scans)?
-
What happens if my cancer doesn’t respond to immunotherapy?
-
Are there signs of immune-related side effects I should watch for?
-
Will I need hormone tests or medications after treatment?
-
How often will I need follow-up appointments after finishing therapy?
-
Is there a survivorship program or support group I can join?
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is immunotherapy?
Immunotherapy is a cancer treatment that helps the immune system recognize and destroy cancer cells.
How does immunotherapy work?
It uses drugs like checkpoint inhibitors to block proteins that stop immune cells from attacking cancer.
How long does immunotherapy stay in the body?
Drugs like pembrolizumab and nivolumab can stay active for weeks, with effects lasting even after treatment ends.
What factors affect how long immunotherapy lasts?
Drug type, dosage, patient metabolism, immune function, and tumor size all play a role.
How long do allergy immunotherapy effects last?
Benefits from allergy shots or drops can last years, especially after 3+ years of treatment.
How is immunotherapy effectiveness monitored?
Doctors use scans, blood tests, and symptom tracking to assess treatment response.
What are common long-term side effects of immunotherapy?
Fatigue, joint pain, skin issues, and hormone imbalances are common and may need ongoing care.
Can immunotherapy effects continue after treatment stops?
Yes, some immune effects and side effects can last for months or even years post-treatment.
What should I ask my doctor before starting immunotherapy?
Ask about duration, possible side effects, monitoring plans, and follow-up care.
Where can I learn more about immunotherapy?
You can watch helpful videos and patient stories on OncoDaily YouTube TV.


