Insulin-like growth factor-1 (IGF-1) has long been implicated in prostate cancer (PCa) pathogenesis and progression, promoting tumor growth through activation of the IGF-1 receptor (IGF-1R). Epidemiologic data link elevated circulating IGF-1 levels with increased prostate cancer risk and mortality. Beyond its mitogenic and anti-apoptotic effects, IGF-1 exerts immunomodulatory and immunosuppressive actions in various tumor settings, potentially explaining the limited efficacy of immune checkpoint blockade (ICB) in prostate cancer.
Earlier studies demonstrated that IGF-1 depletion could restore antigen presentation and CD8⁺ T-cell infiltration in preclinical glioma models, suggesting that IGF-1 may impair anti-tumor immunity by disrupting antigen processing and enhancing immunosuppressive signaling. This study systematically explored the tumor-intrinsic immune effects of IGF-1 in prostate cancer using multi-omics, in vitro, and clinical data.
Study Design and Methods
The authors investigated three prostate cancer cell lines—human androgen receptor (AR)-positive 22Rv1, AR-negative DU145, and murine Myc-CaP—treated with recombinant IGF-1 (30 nM for 24 h).
Comprehensive RNA sequencing, gene set enrichment analysis (GSEA), RT-qPCR, and flow cytometry were used to assess changes in immune-related gene expression, antigen processing, and checkpoint regulation.
Clinical validation employed TCGA Firehose Legacy datasets and multiplex immunofluorescence (mIF) on prostatectomy samples from the ProMPT cohort to correlate IGF-axis activity (serum IGF-1, tumor IGF1/IGFBP5 expression) with PD-L1 expression and immune infiltration.
Key Findings
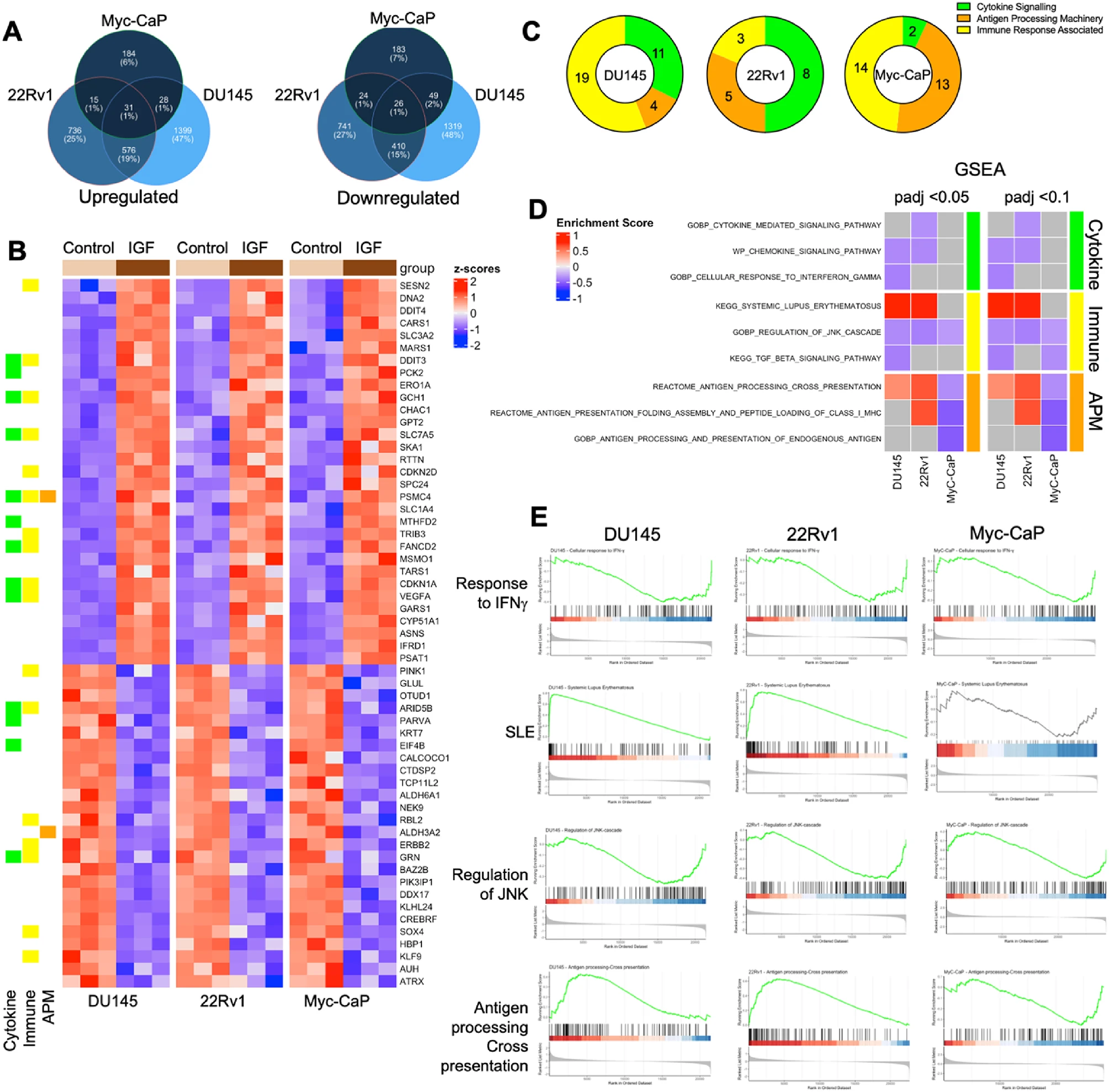
1. IGF-1 globally suppresses immune and cytokine signaling pathways
Transcriptomic profiling revealed that IGF-1 downregulated cytokine signaling, antigen processing and presentation, and interferon-γ–responsive pathways across all models. GSEA confirmed suppression of immune response genes alongside activation of cell-cycle and DNA-repair programs, highlighting a shift toward proliferative and immune-evasive states.
2. Disruption of the antigen processing machinery (APM)
IGF-1 consistently downregulated TAP1, TAP2, ERAP1, and β2-microglobulin (β2M)—key components required for peptide transport and loading onto MHC-I complexes.
Flow cytometry in Myc-CaP cells demonstrated a marked reduction in surface MHC-I (H-2Kq) expression, confirming functional impairment of antigen presentation. This occurred without major changes in HLA-I heavy-chain gene expression, suggesting a defect in peptide processing rather than MHC synthesis.
3. IGF-1 upregulates PD-L1 via the IGF-1R/AKT/ERK signaling cascade
Contrary to its repressive effects on APM genes, IGF-1 significantly induced CD274 (PD-L1) mRNA and surface protein expression in DU145 cells. Pharmacologic blockade of IGF-1R, AKT, or MEK-ERK abrogated this effect, implicating canonical IGF-1 signaling in checkpoint regulation.
This upregulation was not limited to prostate cancer; similar PD-L1 induction was observed in colorectal adenocarcinoma cells with high IGF-1R expression.
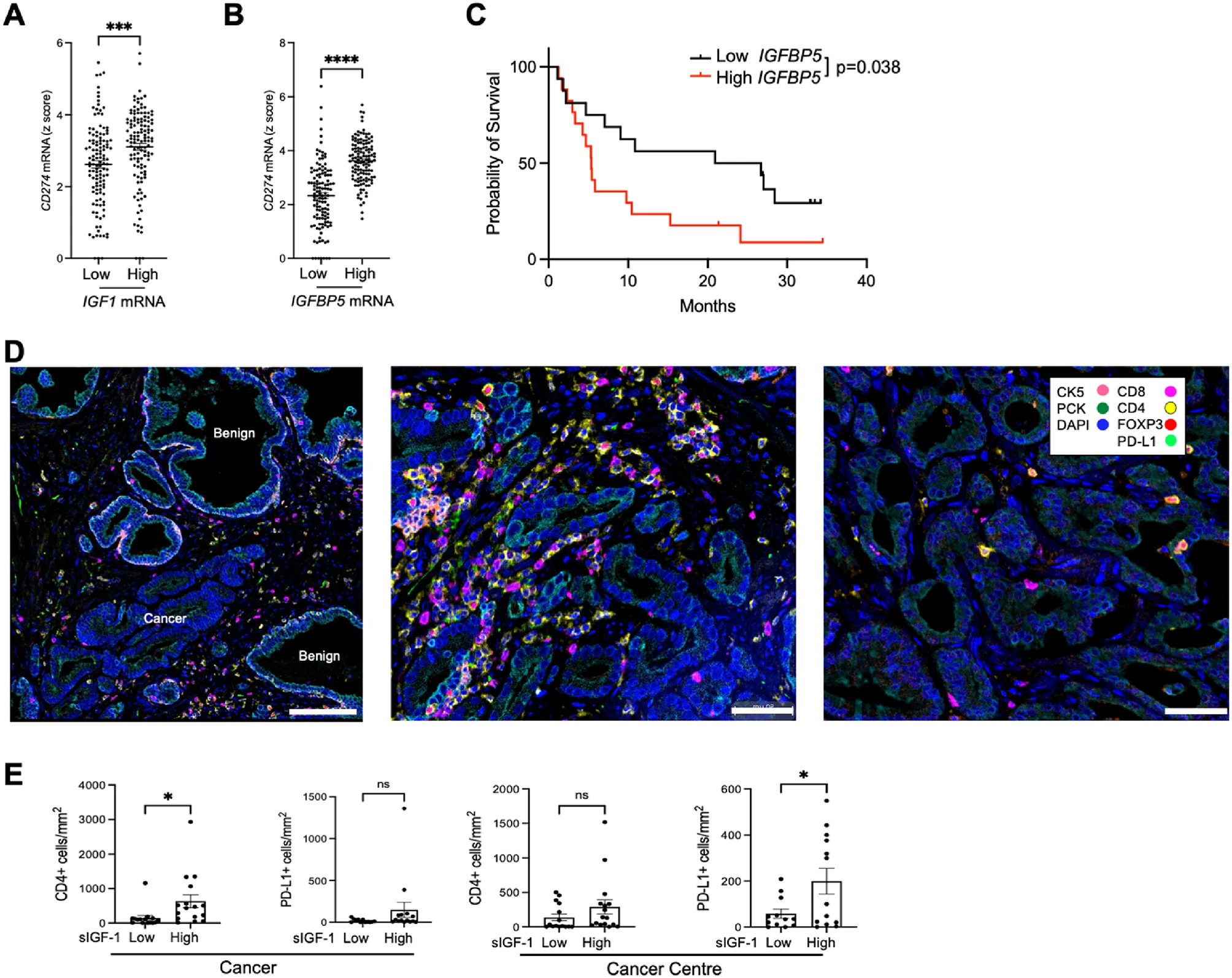
4. Clinical validation: high IGF-axis activity correlates with PD-L1 expression and poor immune infiltration
Analysis of TCGA prostate adenocarcinoma data (n = 498) showed that tumors in the highest quartiles of IGF1 or IGFBP5 expression had significantly higher CD274 (PD-L1) levels.
In prostatectomy samples, patients with high serum IGF-1 exhibited increased PD-L1⁺ epithelial and stromal cells and more CD4⁺ T-cell infiltration, though CD8⁺ T-cell and FOXP3⁺ Treg numbers were unchanged.
Furthermore, high IGFBP5 expression correlated with shorter survival after CTLA-4 blockade in a melanoma cohort, suggesting that IGF-axis activation may contribute to ICB resistance across tumor types.
Mechanistic Interpretation
The study identifies a dual immunosuppressive role for IGF-1 in prostate cancer:
- Antigen presentation impairment: IGF-1 disrupts peptide transport and processing (via TAP1/2, ERAP1, β2M downregulation), reducing surface MHC-I expression and impairing CD8⁺ T-cell recognition.
- Checkpoint activation: Simultaneously, IGF-1 enhances PD-L1 expression through IGF-1R-dependent activation of AKT and ERK pathways, reinforcing an immune-evasive tumor phenotype.
This coordinated suppression of antigen presentation and activation of PD-L1 expression defines an IGF-1-driven immune escape program in prostate cancer.
Clinical and Translational Implications
These findings illuminate a novel IGF-1–mediated immunosuppressive axis that may underlie the refractory nature of prostate cancer to PD-1/PD-L1 blockade. Therapeutic inhibition of IGF-1R or downstream AKT/ERK signalingcould therefore potentiate immune checkpoint therapy by restoring MHC-I presentation and reducing PD-L1 expression.
Given that high serum IGF-1 and IGFBP5 expression correlate with elevated PD-L1 and poor post-ICB outcomes, these parameters may serve as biomarkers of immune exclusion and predictors of immunotherapy resistance in prostate cancer and potentially other malignancies.
Conclusion
The study demonstrates that IGF-1 orchestrates prostate cancer immune evasion by concurrently suppressing antigen presentation machinery and upregulating PD-L1 via IGF-1R/AKT/ERK signaling. These results establish the IGF axis as a key regulator of tumor immune escape and support the rationale for combination strategies targeting IGF-1R signaling with immune checkpoint blockade in immunotherapy-resistant prostate cancer.
Can You Read All Article Here