HERIZON-GEA-01 is a global, randomized phase 3 trial, and updated results were presented at the ASCO Gastrointestinal Cancers Symposium, evaluating zanidatamab-based regimens compared with trastuzumab plus chemotherapy as first-line treatment for patients with HER2-positive metastatic gastroesophageal adenocarcinoma (mGEA). The study assesses the clinical impact of dual HER2 targeting, with or without immune checkpoint inhibition, in a population where outcomes with current standard-of-care therapy remain limited.
Beckground
Outcomes with current standard-of-care therapy for first-line HER2-positive mGEA remain modest, with median progression-free survival typically under one year and median overall survival under two years. Zanidatamab is a dual HER2-targeted bispecific IgG1-like antibody that binds extracellular domains 2 and 4 of HER2 in a trans configuration. This biparatopic binding enables receptor clustering and enhanced HER2 internalization, leading to reduced downstream signaling and promotion of immune-mediated cytotoxic mechanisms, including antibody-dependent cellular cytotoxicity, phagocytosis, and complement-dependent cytotoxicity. Tislelizumab is a PD-1 inhibitor engineered to minimize Fcγ receptor binding on macrophages. Prior phase 2 studies of zanidatamab-based combinations demonstrated promising activity and a tolerable safety profile in HER2-positive mGEA, supporting evaluation in a phase 3 setting.
Study Design
HERIZON-GEA-01 is a randomized, global, phase 3 trial enrolling adults with previously untreated, unresectable locally advanced, recurrent, or metastatic HER2-positive gastroesophageal adenocarcinoma. Key eligibility criteria included HER2 IHC 3+ or IHC 2+/ISH+ status, ECOG performance status 0–1, and no prior HER2-targeted therapy or immunotherapy in any setting.
A total of 914 patients were randomized in a 1:1:1 ratio to one of three treatment arms: trastuzumab plus chemotherapy, zanidatamab plus chemotherapy, or zanidatamab plus tislelizumab plus chemotherapy. Chemotherapy backbones included CAPOX or FP. Treatment was continued until disease progression, death, or unacceptable toxicity, with chemotherapy allowed to be discontinued after six cycles per protocol.

The trial has dual primary endpoints of progression-free survival and overall survival, analyzed in the intent-to-treat population using a fixed-sequence testing procedure. Secondary endpoints included confirmed objective response rate, duration of response, and safety.
Patient Characteristics and Disposition
Baseline demographics and disease characteristics were well balanced across the three treatment arms. Median age was approximately 63 years, with the majority of patients having metastatic disease at enrollment. HER2 IHC 3+ disease was present in over 80% of patients across arms, and PD-L1 tumor area positivity scores were distributed across both <1% and ≥1% categories.
At the time of analysis, median follow-up exceeded two years. Ongoing treatment rates ranged from 12% to 29% across arms, with disease progression being the most common reason for treatment discontinuation.
Results
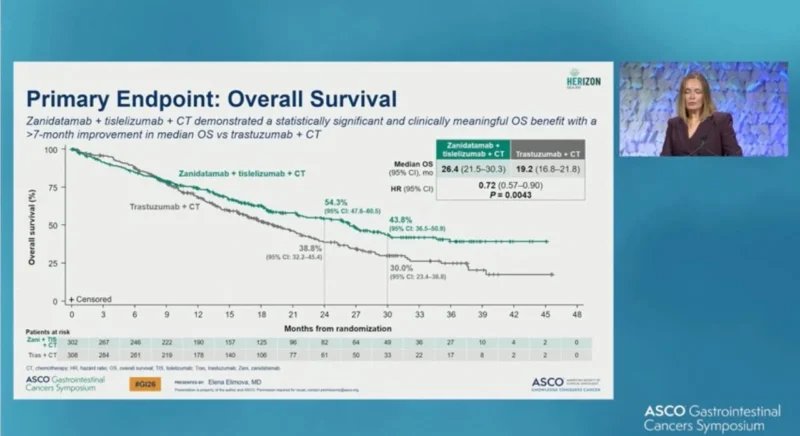
At the interim analysis, a strong trend toward improved overall survival was observed with zanidatamab plus chemotherapy compared with trastuzumab plus chemotherapy. Median overall survival was 26.4 months with zanidatamab and tislelizumab plus chemotherapy versus 19.2 months with trastuzumab plus chemotherapy, corresponding to a hazard ratio of 0.72 (95% CI, 0.57–0.90; P = 0.0043). Overall survival improvements were observed across major prespecified subgroups, including geographic regions and PD-L1 tumor area positivity scores.
Progression-free survival improvements were generally consistent across key prespecified subgroups. Forest plot analyses demonstrated hazard ratios favoring zanidatamab-containing arms across most categories, including age, geographic region, ECOG performance status, anatomical subtype, HER2 status, and PD-L1 expression.
Antitumor Activity
Zanidatamab-containing regimens demonstrated deeper and more durable responses compared with trastuzumab plus chemotherapy. Confirmed objective response rates in patients with measurable disease were 70.7% for zanidatamab plus tislelizumab plus chemotherapy and 69.6% for zanidatamab plus chemotherapy, compared with 65.7% for trastuzumab plus chemotherapy. Complete response rates were higher in the zanidatamab-containing arms.
Median duration of response was 20.7 months with zanidatamab plus tislelizumab plus chemotherapy and 14.3 months with zanidatamab plus chemotherapy, compared with 8.3 months with trastuzumab plus chemotherapy.
Safety
The safety profile was consistent with known toxicities of the individual agents and chemotherapy backbones. The most common treatment-related adverse event across all arms was diarrhea. Other common events occurring in at least 20% of patients included nausea, vomiting, decreased appetite, anemia, peripheral sensory neuropathy, weight loss, infusion-related reactions, and cytopenias. Rates of grade ≥3 events were reported across arms and were manageable with standard supportive measures.
Conclusion
Data presented at ASCO GI from HERIZON-GEA-01 demonstrate that zanidatamab-based regimens show consistent improvements in efficacy outcomes compared with trastuzumab plus chemotherapy in first-line HER2-positive metastatic gastroesophageal adenocarcinoma, with deeper and more durable responses observed. Overall survival analyses show a strong trend favoring zanidatamab plus chemotherapy, with continued follow-up ongoing.
For more information click here.


