Hepatocellular carcinoma (HCC) remains one of the leading causes of cancer-related mortality worldwide. While curative options such as liver resection, transplantation, or local ablation offer the best survival outcomes, the majority of patients present with advanced or unresectable disease and are treated with systemic therapy. The emergence of immune checkpoint inhibitors (ICIs)—targeting PD-1, PD-L1, and CTLA-4—has transformed HCC management, achieving response rates up to 30–40% with modern immunotherapy combinations such as atezolizumab plus bevacizumab. These responses raise the possibility of “curative treatment conversion” (CTC)—where patients initially treated with immunotherapy become eligible for curative interventions after tumor downstaging.
This study, published in Liver Cancer in 2025, represents the largest real-world analysis to date investigating curative treatment following immunotherapy in HCC using the U.S. National Cancer Database (NCDB), which includes over 70% of all newly diagnosed cancer cases in the United States.
Study Design and Methods
The investigators analyzed 4,329 patients with HCC who received first-line immunotherapy between 2017 and 2020. Patients were divided into two groups:
- CTC group: Patients who underwent curative treatment after immunotherapy (resection, transplantation, or local ablation).
- Non-CTC group: Patients who continued immunotherapy without subsequent curative intervention.
The primary goal was to identify factors associated with curative treatment conversion and assess the impact on overall survival (OS). Statistical analyses included multivariable Cox regression, propensity score (PS) matching, and inverse probability of treatment weighting (IPTW) to minimize confounding.
Key Results
In this large national analysis, only 3.2% (138 of 4,329) patients with hepatocellular carcinoma (HCC) who received first-line immunotherapy went on to receive curative treatment. The median time from the start of immunotherapy to curative intervention was 3 months, reflecting relatively rapid downstaging in selected responders. Among those who achieved curative conversion, liver resection was the most common procedure (54.3%), followed by local ablation(25.4%) and liver transplantation (20.3%).
Patients who became eligible for curative therapy after immunotherapy tended to have more favorable baseline features. They were younger (median age 63 vs. 66 years, p = 0.004), had smaller tumor size (median 57 mm vs. 75 mm, p = 0.002), and generally presented with lower T, N, and M stages compared with those who did not undergo curative treatment. Importantly, they were more likely to have received care at academic centers (78% vs. 48%, p < 0.001), highlighting the role of specialized institutions in facilitating curative conversion.
On multivariable logistic regression, several factors emerged as independent predictors of receiving curative treatment following immunotherapy. These included lower tumor stage (T1–T3), absence of nodal metastasis (odds ratio [OR] 0.16; 95% CI, 0.02–0.56), and treatment at an academic health system (OR 3.40; 95% CI, 1.68–7.38). Collectively, these findings suggest that younger age, limited disease burden, and access to academic multidisciplinary care are key determinants for achieving curative conversion after immunotherapy in HCC.
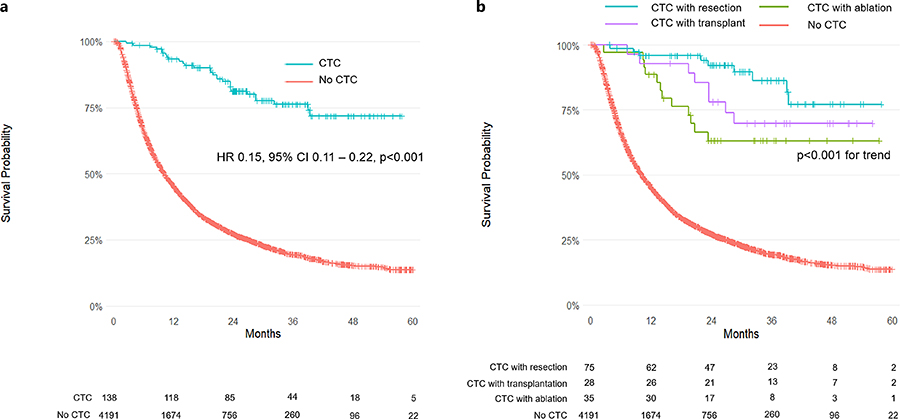
Survival Outcomes
Curative treatment after immunotherapy was associated with remarkably improved survival:
- Median OS: Not reached in the CTC group vs. 10 months in non-CTC group
- Unadjusted HR: 0.15 (95% CI: 0.11–0.22; p < 0.001)
- PS-matched HR: 0.20 (95% CI: 0.13–0.30; p < 0.001)
- IPTW-adjusted HR: 0.19 (95% CI: 0.11–0.31; p < 0.001)
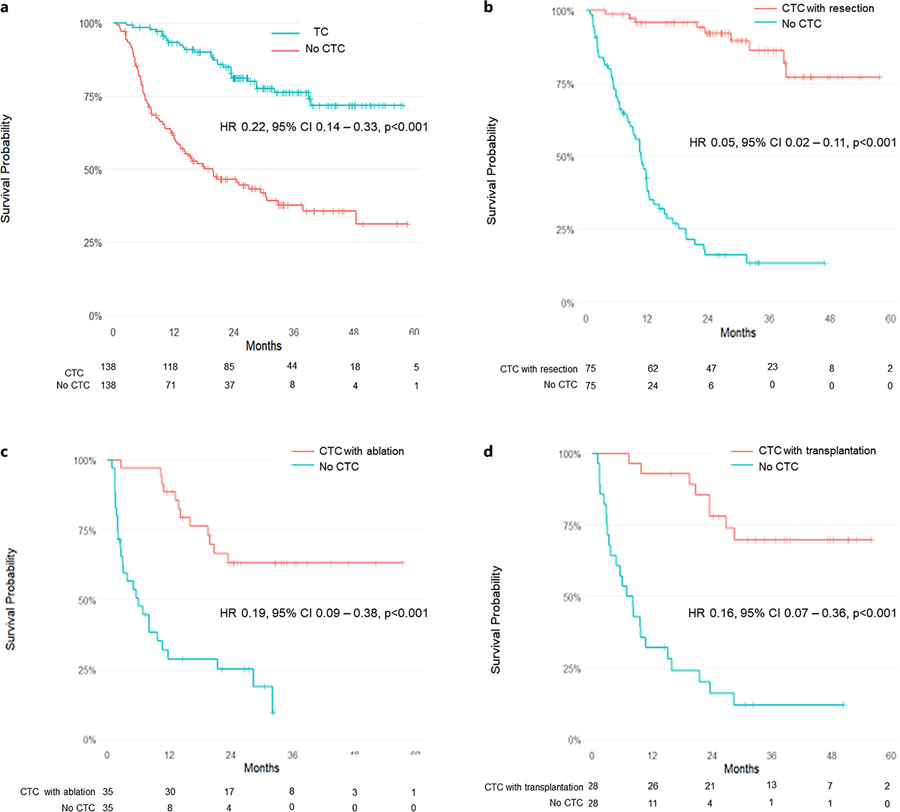
Among individual curative strategies:
- Resection: HR 0.18 (95% CI: 0.08–0.40; p < 0.001)
- Transplantation: HR 0.28 (95% CI: 0.09–0.87; p = 0.03)
- Ablation: HR 0.21 (95% CI: 0.05–0.86; p = 0.03)
The median follow-up was 33 months for the CTC group and 29 months for the non-CTC group. The survival benefit persisted across all T stages and remained independent of institutional setting.
Academic Center Impact
Care at academic health centers was a strong independent predictor of both:
- Curative treatment receipt (OR 3.40; p = 0.001)
- Improved overall survival (HR 0.70; p < 0.001)
Even when stratified by institutional type, the benefit of CTC remained significant in both academic and nonacademic settings, suggesting that academic institutions provide broader infrastructure, multidisciplinary expertise, and access to curative interventions that enhance outcomes.
Discussion and Clinical Implications
This study demonstrates that curative treatment following immunotherapy, though rare (3.2%), is associated with exceptional long-term survival in HCC. These findings suggest that a subset of patients with advanced disease can be downstaged to potentially curative therapy after effective immune checkpoint blockade.
The authors emphasize the importance of multidisciplinary evaluation and early referral to academic centers, where curative options such as resection, ablation, or transplantation can be pursued once immunotherapy response is achieved.
Moreover, emerging data from Japan and the U.S. reinforce that atezolizumab plus bevacizumab can yield high response rates (up to 44%), enabling curative conversion in 8–35% of previously unresectable cases. These real-world findings validate the curative conversion concept and underscore the expanding role of immunotherapy in bridging, downstaging, and neoadjuvant strategies for HCC.
You Can Read All Article Here