Gastric and gastroesophageal junction cancers (GC/GEJC) remain among the most challenging malignancies to treat, with limited long-term survival despite advances in targeted and immunotherapies. While FGFR2 amplification and overexpression have been recognized as potential therapeutic targets, recent research has revealed that not all FGFR2 alterations behave equally.
FGFR2 exists in two major splice variants — FGFR2-IIIb and FGFR2-IIIc — which differ in tissue expression, ligand affinity, and oncogenic potential. Emerging evidence suggests that FGFR2-IIIc, typically expressed in mesenchymal tissues, may promote tumor invasion and treatment resistance. To clarify its clinical significance, investigators performed an in-depth transcriptomic and clinical analysis of FGFR2 isoforms in patients with advanced GC/GEJC, aiming to define their prognostic and therapeutic implications.
Study Design
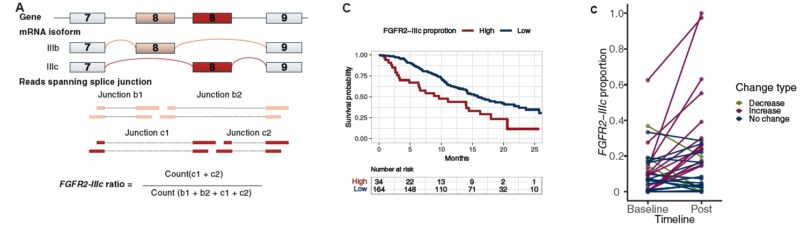
The analysis included 235 patients with advanced GC/GEJC enrolled in the MONSTAR-SCREEN-2 study (UMIN000043899). Whole-transcriptome sequencing (WTS) was performed to quantify FGFR2-IIIb and FGFR2-IIIc splice variants, and immunohistochemistry (IHC) was used to assess FGFR2 protein expression. Patients were divided into high-IIIc and low-IIIc groups based on a validated threshold of 0.28 for FGFR2-IIIc proportion. The primary endpoint was overall survival, while secondary analyses included treatment response, molecular correlates, and treatment-induced isoform switching.

Results
The results, published in ESMO Open (November 2025), showed that a high FGFR2-IIIc proportion was strongly associated with poor prognosis. Among 209 patients with detectable FGFR2 expression, those with high FGFR2-IIIc levels (>0.28) had a median overall survival of 9.6 months compared with 16.0 months in the low-IIIc group (hazard ratio 2.08, 95% CI 1.33–3.26, P < 0.001). Multivariable analysis confirmed FGFR2-IIIc as an independent prognostic factor for mortality, outperforming total FGFR2 expression levels. Validation using The Cancer Genome Atlas (TCGA) dataset supported these findings, confirming shorter survival in the high-IIIc cohort (HR 1.86, P = 0.0079).
No significant differences in response rate were observed between IIIc groups across various therapies, but longitudinal analysis revealed a significant rise in FGFR2-IIIc expression following treatment. Among 33 patients with paired pre- and post-treatment samples, 39% exhibited treatment-induced increases in FGFR2-IIIc proportion (P = 0.0085), suggesting adaptive isoform switching as a potential mechanism of resistance. Interestingly, all tumors with FGFR2 amplification or high overall FGFR2 expression belonged exclusively to the low-IIIc group, indicating that existing FGFR2-targeted therapies, which focus on the IIIb isoform, may not benefit patients with FGFR2-IIIc-dominant tumors.
Molecular analysis further revealed that high-IIIc tumors were enriched in epithelial-to-mesenchymal transition (EMT) and myogenesis pathways, consistent with a more invasive and aggressive phenotype. Differential gene expression analysis identified EPHA7 and MTTP as the most upregulated genes in high-IIIc cancers, both previously linked to tumor progression and ferroptosis resistance. Transcriptomic analysis revealed reduced expression of the splicing regulator ESRP1, which controls FGFR2 splicing. Coordinated dysregulation of its target genes, including CD44 and ENAH, further supported a shift toward mesenchymal transition.
Experts’ Perspectives
The publication has drawn attention within the Japanese oncology community.
Dr. Daisuke Kotani, Chief Physician at the Department of Gastrointestinal Oncology, National Cancer Center Hospital East, shared:
“Our colleagues Dr. Hashimoto and the MONSTAR-3 team just published in ESMO Open! FGFR2-IIIc isoform detection reveals prognostic prevalence and a functional link to mesenchymal transition in gastric and gastroesophageal cancers.”
Dr. Kohei Shitara, Director of the same department and co-author of the paper, added:
“We show that a high FGFR2-IIIc to IIIb isoform ratio in gastric and GEJ cancers correlates with poor prognosis and epithelial–mesenchymal transition. Further analysis in bemarituzumab-treated tumors is ongoing.”
Their reflections underscore how isoform-level profiling is reshaping the biological understanding of FGFR2 and may guide the next generation of targeted therapies in gastric cancer.
Conclusion
This comprehensive transcriptomic analysis identifies FGFR2-IIIc as a powerful prognostic biomarker and uncovers treatment-induced isoform switching as a potential mechanism of therapeutic resistance in advanced GC/GEJC. These findings highlight an unmet clinical need for the development of FGFR2-IIIc-specific inhibitors and for integrating isoform-level molecular profiling into personalized treatment strategies.
By distinguishing FGFR2 isoforms through whole-transcriptome sequencing, clinicians may be able to better predict outcomes and guide targeted therapy selection. The discovery of FGFR2-IIIc–driven resistance underscores the importance of continuous molecular monitoring and the evolution of precision oncology in gastric and gastroesophageal cancers.
You can also read about Pemigatinib (Pemazyre): Uses in Cancer, Side effects, Dosage, Expectation on OncoDaily.