Gastroesophageal adenocarcinoma (GEA) remains one of the leading causes of cancer mortality worldwide, with limited survival despite advances in immunotherapy. While PD-1 blockade combined with chemotherapy has improved outcomes, median overall survival typically remains around 13–15 months.
TIGIT inhibition represents a next-generation immune checkpoint strategy aimed at enhancing antitumor T-cell activation. Domvanalimab, an Fc-silent anti-TIGIT antibody, combined with the anti-PD-1 agent zimberelimab and chemotherapy, is being explored to further improve first-line outcomes in advanced or unresectable GEA.
Study Design and Regimen
The EDGE-Gastric trial (NCT05329766) is a global, multi-arm, Phase 2 study designed to evaluate the safety and efficacy of various combinations of the Fc-silent anti-TIGIT antibody domvanalimab and the anti-PD-1 monoclonal antibody zimberelimab in patients with locally advanced unresectable or metastatic gastric, gastroesophageal junction (GEJ), or esophageal adenocarcinoma.
In Arm A1, treatment-naïve patients received domvanalimab 1600 mg intravenously every four weeks, zimberelimab 480 mg intravenously every four weeks, and FOLFOX chemotherapy (oxaliplatin, leucovorin, and 5-fluorouracil) administered every two weeks. The study, conducted in partnership with Gilead Sciences, aims to assess key efficacy endpoints including overall survival (OS), progression-free survival (PFS), and objective response rate (ORR), alongside a detailed evaluation of safety and tolerability.
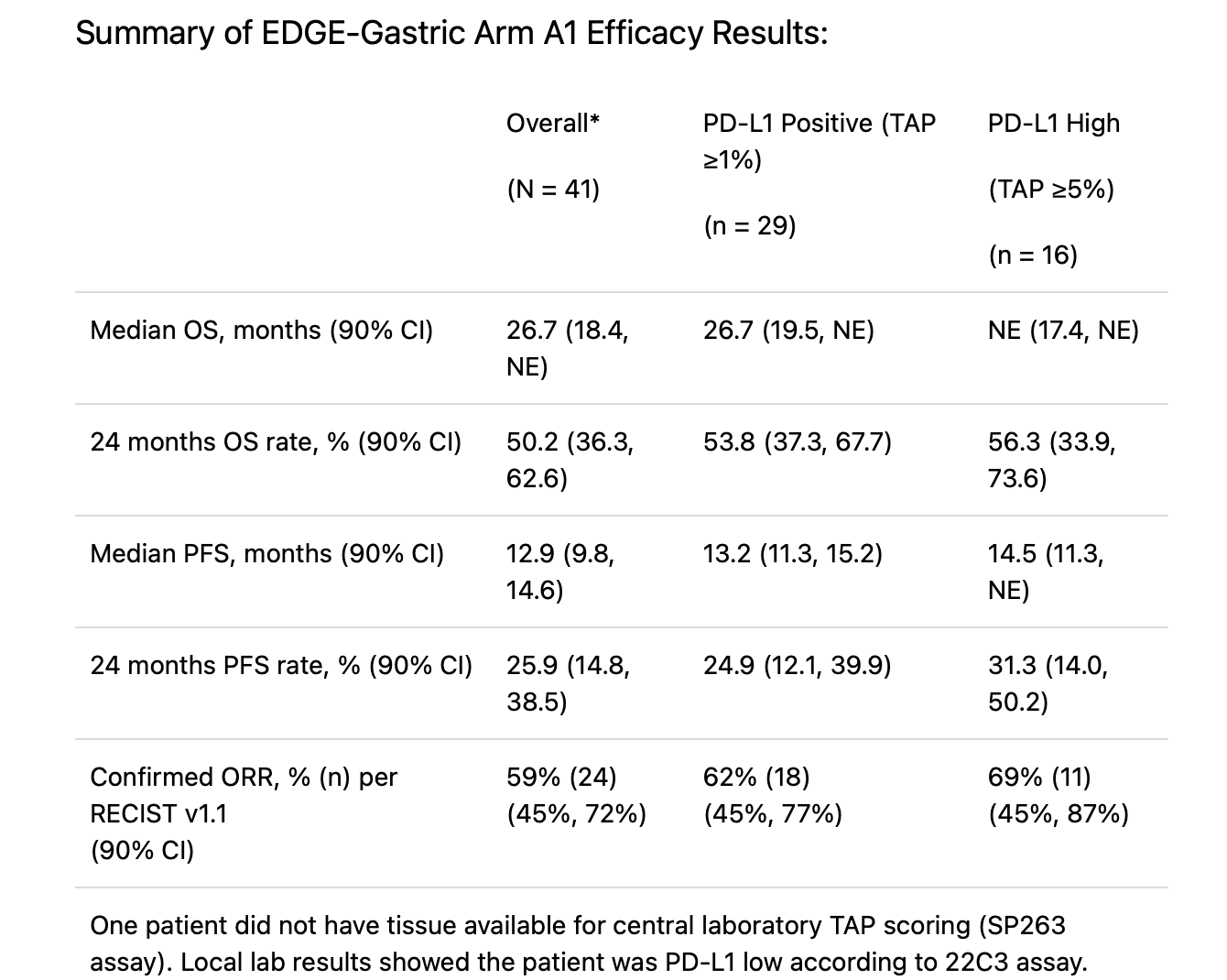
Key Results and Efficacy
The EDGE-Gastric Phase 2 trial demonstrated encouraging efficacy for the combination of domvanalimab, zimberelimab, and FOLFOX chemotherapy in patients with advanced or unresectable gastroesophageal adenocarcinoma. In the overall study population of 41 patients, the median overall survival (OS) reached 26.7 months(90% CI: 18.4–not estimable), with a 24-month OS rate of approximately 50%. Similar results were observed across PD-L1 subgroups: patients with PD-L1–positive tumors (TAP ≥1%) had a median OS of 26.7 months, while those with high PD-L1 expression (TAP ≥5%) had not yet reached median OS at the time of analysis.
The median progression-free survival (PFS) was 12.9 months (90% CI: 9.8–14.6), and the 24-month PFS rate was approximately 26%. The confirmed objective response rate (ORR) was 59% per RECIST v1.1, increasing to 62%among PD-L1–positive and 69% among PD-L1–high tumors.
Overall, these results indicate durable clinical benefit across PD-L1 expression levels, suggesting that dual checkpoint blockade with anti-TIGIT and anti-PD-1 antibodies combined with chemotherapy may provide a meaningful improvement over current first-line PD-1–based regimens in advanced gastroesophageal cancer.
Safety and Tolerability
- No unexpected safety signals reported at data cutoff (March 2025).
- Immune-mediated adverse events (AEs): Occurred in 9 patients (22%)
- Infusion-related reactions: 7% (3 patients)
- In earlier reports (ASCO 2024), grade ≥3 adverse events (AEs) were pervasive, but toxicity was consistent with known profiles of PD-1 + chemotherapy.
Caveats and Considerations
While the results from the EDGE-Gastric Phase 2 trial are promising, several important limitations should be considered when interpreting the findings. The small sample size (n = 41) limits statistical precision, resulting in wide confidence intervals and less stable survival estimates. Moreover, as a nonrandomized, single-arm study, the trial is inherently susceptible to selection bias, and favorable baseline characteristics among enrolled patients may have contributed to the observed outcomes.
The overall survival data are still maturing, and longer follow-up may influence the current median OS estimates. Additionally, because there was no control arm, comparisons with standard regimens such as nivolumab plus chemotherapy remain indirect. These limitations underscore the need for confirmation in randomized, controlled settings, such as the ongoing Phase 3 STAR-221 trial, which will provide definitive evidence regarding the true efficacy and comparative benefit of the domvanalimab–zimberelimab–chemotherapy combination.

You Can Read About 10 ongoing Clinical Trials on Immunotherapy in Gastric Cancer


