Gastric cancer with peritoneal dissemination is associated with poor prognosis and limited therapeutic options, particularly after progression on fluoropyrimidine-based first-line therapy. Although ramucirumab combined with solvent-based paclitaxel is the established second-line standard of care, nanoparticle albumin-bound paclitaxel has been hypothesised to offer pharmacologic advantages through albumin-mediated drug delivery, providing a pharmacological rationale for evaluation in this setting. However, prospective randomised data in this specific population have been lacking.
To address this gap, the West Japan Oncology Group conducted the P-SELECT (WJOG10617G) randomized phase II trial comparing solvent-based paclitaxel plus ramucirumab with nab-paclitaxel plus ramucirumab in patients with pretreated gastric cancer and peritoneal dissemination, with a prespecified translational analysis focused on stromal biomarkers. This study was published in February 2026 in eClinicalMedicine.
Title: Solvent-based or nab-paclitaxel plus ramucirumab for pretreated gastric cancer with peritoneal dissemination and prespecified biomarker analysis (P-SELECT/WJOG10617G): a randomised phase 2 trial in Japan
Authors: Kenro Hirata, Yasuo Hamamoto, Hirokazu Shoji, Hiroki Hara, Chihiro Kondoh, Hisateru Yasui, Takeshi Kajiwara, Eishi Baba, Takayuki Ando, Naotoshi Sugimoto, Hisato Kawakami, Hiroo Katsuya, Michitaka Nagase, Yoshiyuki Yamamoto, Kenichi Yoshimura, Masahiko Ando, Chiyo K. Imamura, Kentaro Yamazaki, Shuichi Hironaka, and Kei Muro.
Read about Gastric Cancer Remission Rate on OncoDaily.
Methods
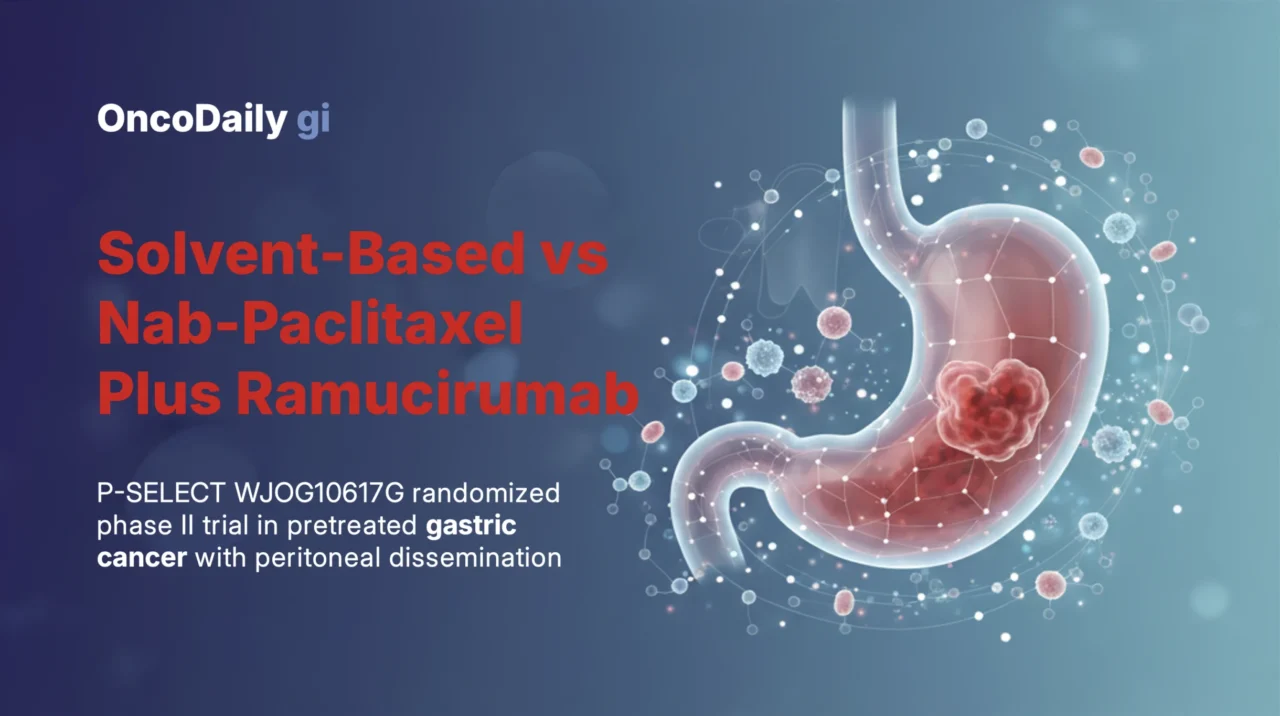
P-SELECT was a prospective, open-label, multicentre, randomized phase II trial conducted at 58 centres in Japan within the West Japan Oncology Group. Eligible patients had histologically confirmed gastric or gastro-oesophageal junction adenocarcinoma with peritoneal dissemination and were refractory or intolerant to fluoropyrimidine-based first-line therapy.
Patients were randomized 1:1 to receive ramucirumab (8 mg/kg on days 1 and 15) combined with either weekly solvent-based paclitaxel (80 mg/m² on days 1, 8, and 15) or weekly nab-paclitaxel (100 mg/m² on days 1, 8, and 15) in 28-day cycles. Randomisation was stratified by institution, ECOG performance status, and ascites severity.
The primary endpoint was overall survival (OS). Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), time to treatment failure, ascites response and control, safety, patient-reported peripheral neuropathy, pharmacokinetics of ramucirumab, and prespecified tissue biomarker analyses. Stromal caveolin-1 (Cav-1) and SPARC expression were assessed by immunohistochemistry on archival tumour specimens by a blinded pathologist. The data cutoff was January 27, 2021. This trial was registered in the Japan Registry of Clinical Trials (jRCTs031180022).
Results
Between October 1, 2018, and January 27, 2020, 105 patients were enrolled and randomized to sb-PTX plus ramucirumab (n = 53) or nab-PTX plus ramucirumab (n = 52). Median follow-up was 18.1 months. Baseline characteristics were generally balanced, with diffuse-type histology predominating and all patients presenting with peritoneal dissemination.
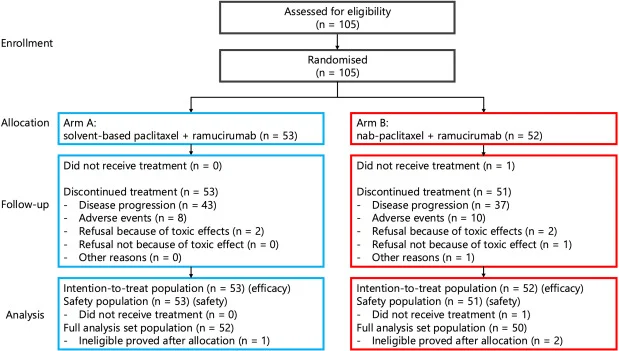
Efficacy outcomes showed no significant difference between treatment arms:
- Median OS was 8.1 months with sb-PTX plus ramucirumab and 7.2 months with nab-PTX plus ramucirumab (HR 0.96; 95% CI 0.62–1.48; P = 0.631).
- Median PFS was 5.1 months versus 3.9 months, respectively (HR 0.97; 95% CI 0.64–1.45; P = 0.893).
- ORR among patients with measurable disease was 20.7% vs 20.0%, and DCR in the intention-to-treat population was 77.4% vs 63.5%, respectively.
Safety profiles were broadly comparable. Grade ≥3 peripheral sensory neuropathy occurred more frequently in the nab-PTX arm, whereas febrile neutropenia was more common in the sb-PTX arm. Relative dose intensity of paclitaxel declined over time in both arms and was overall lower with nab-PTX. Ramucirumab trough concentrations were similar between arms, indicating comparable exposure.
Biomarker analysis identified a treatment-specific association. In the nab-PTX + ramucirumab arm, OS and PFS improved stepwise with increasing stromal Cav-1 expression (P = 0.007 and P = 0.012, respectively), whereas no such association was observed in the sb-PTX arm. SPARC expression, assessed in both stromal and tumour compartments, showed no association with clinical outcomes in either treatment group.
Conclusion
The P-SELECT trial did not demonstrate superior efficacy of nab-paclitaxel plus ramucirumab compared with solvent-based paclitaxel plus ramucirumab in patients with gastric cancer and peritoneal dissemination and did not meet the prespecified threshold for a promising regimen under its phase II screening design.
However, the consistent association between high stromal caveolin-1 expression and improved outcomes with nab-paclitaxel suggests a potential predictive role for this biomarker. While these findings are not practice-changing, they provide a biologically grounded rationale for future prospective validation and underscore the importance of tumour microenvironment–driven treatment selection in this high-risk population.
The full article is available in eClinicalMedicine.