Metastatic gastric cancer (mGC) remains a lethal disease, and while systemic therapy has gradually extended survival in clinical practice, outcomes are still poor overall. Immune checkpoint inhibitors (ICIs), particularly PD-1 blockade, have improved outcomes in multiple solid tumors, and nivolumab is an established later-line option in mGC based on prior randomized evidence.
A growing body of work suggests that circadian biology can shape immune function—immune-cell trafficking, antigen presentation, cytokine release, and effector activation fluctuate over a 24-hour cycle. “Chronotherapy” applies this concept by aligning treatment delivery with the body’s internal clock to potentially improve efficacy and safety. However, until this analysis, there had been no dedicated report in mGC evaluating whether nivolumab infusion timingcorrelates with outcomes.
Study Design and Methods
This was a single-center, retrospective study of patients with unresectable advanced or recurrent gastric/GEJ adenocarcinoma treated with nivolumab monotherapy as third-line or later therapy between December 2014 and December 2022.
- Treatment: nivolumab 3 mg/kg or 240 mg every 2 weeks, or 480 mg every 4 weeks.
- Eligibility highlights: age ≥20, ECOG PS 0–2, ≥2 prior regimens including fluoropyrimidine + taxane, and at least two nivolumab infusions.
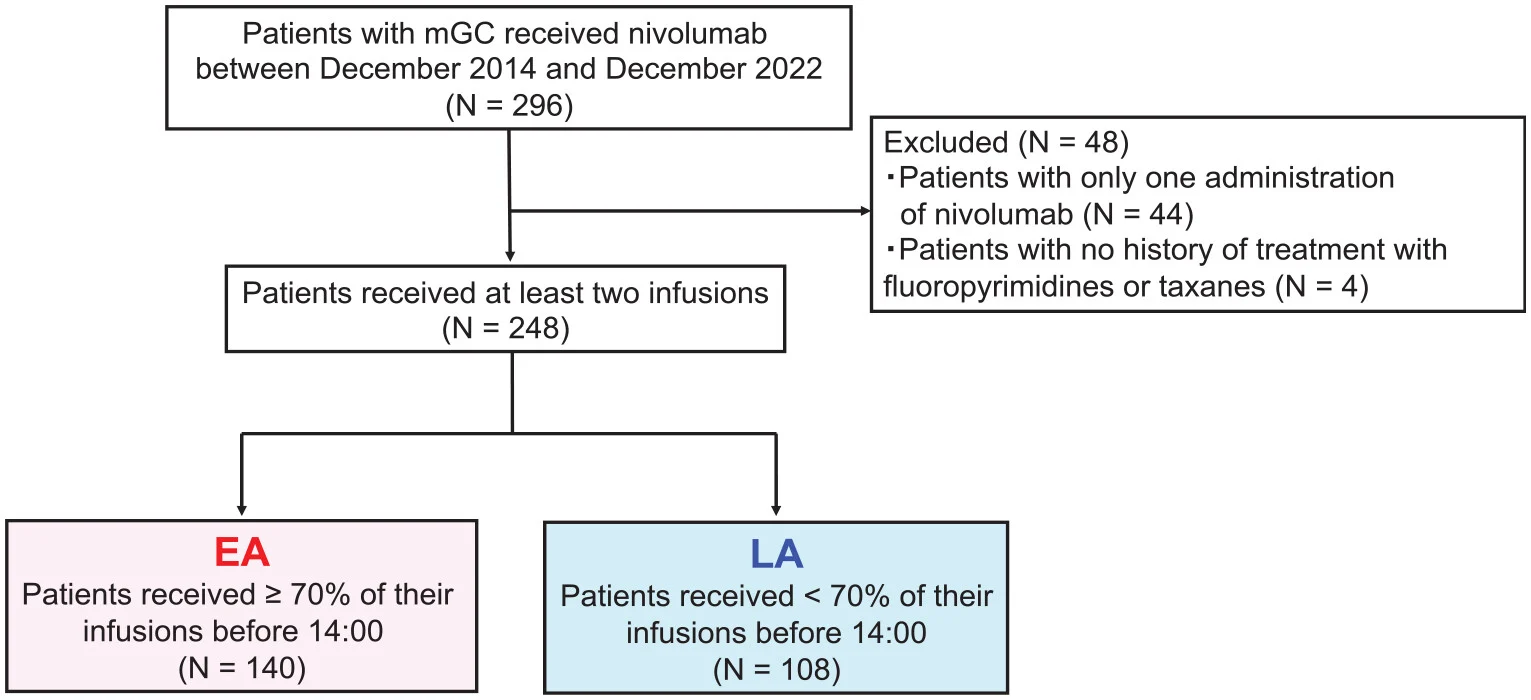
Exposure definition (timing groups)
Infusion start times were pulled from medical records:
- Early infusion group (EA): ≥70% of infusions before 14:00
- Late infusion group (LA): <70% of infusions before 14:00
The 14:00 cutoff was chosen because it matched the most frequent infusion time window (13:30–14:00) and aligned with a prior chronotherapy report design.
Results
Between December 2014 and December 2022, 296 patients received nivolumab, of whom 248 met the eligibility criteria for analysis. Patients were divided according to infusion timing into an early administration (EA) group (n = 140) and a late administration (LA) group (n = 108). Overall, baseline clinicopathologic characteristics were well balanced between the two groups. However, patients in the EA group showed slightly more favorable inflammatory profiles, with a higher proportion of patients having a neutrophil-to-lymphocyte ratio (NLR) <2.4 (59% vs 38%) and a trend toward lower modified Glasgow prognostic scores (mGPS). As expected, the median infusion start time differed substantially between groups (11:50 in the EA group vs 14:22 in the LA group).
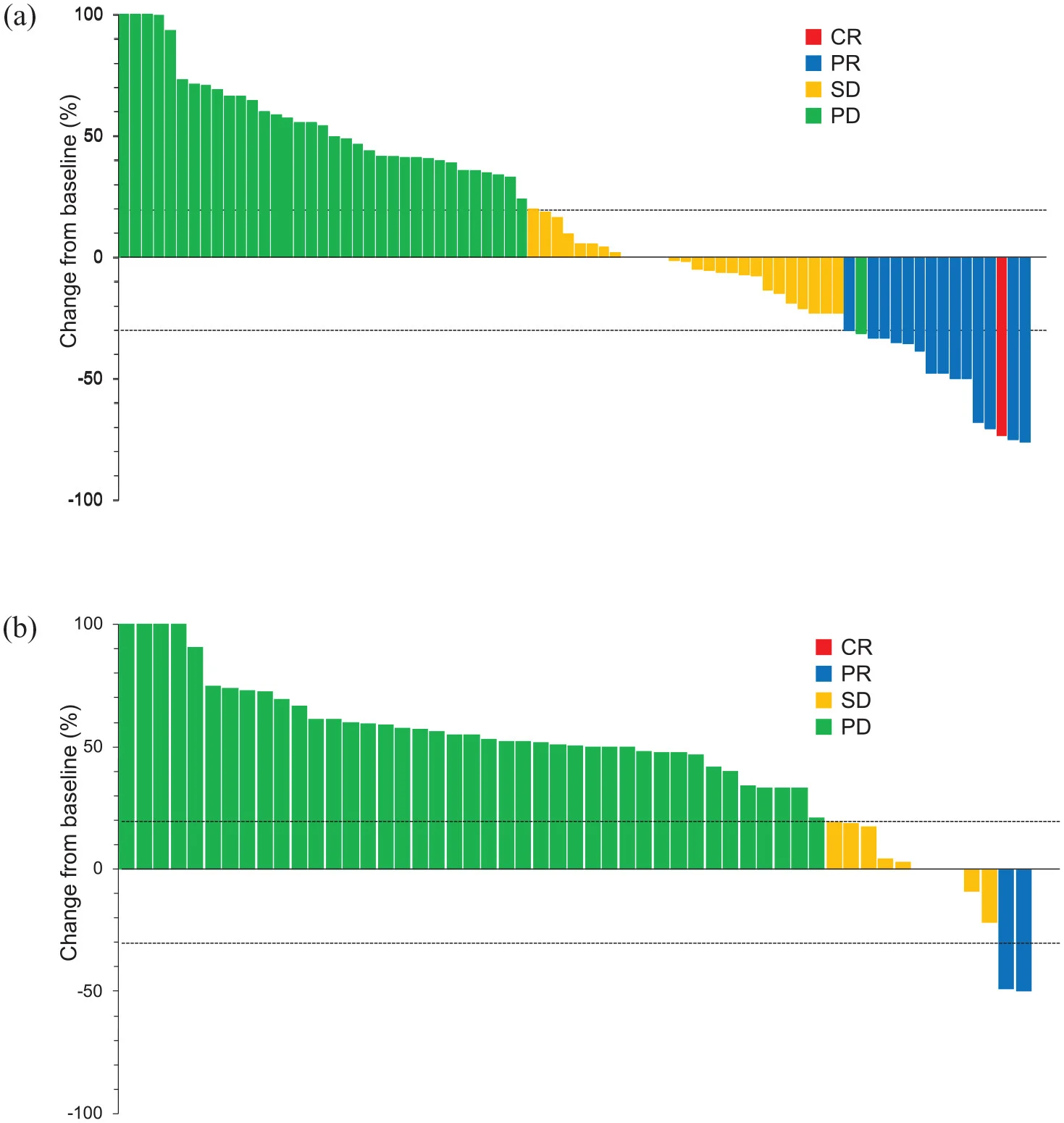
Tumor response
Among the 149 patients with measurable disease, treatment efficacy differed markedly by infusion timing. The objective response rate (ORR) was significantly higher in the EA group (17%) compared with the LA group (3%). Similarly, the disease control rate (DCR) was more than doubled in the EA group (47% vs 20%). In contrast, progressive disease was substantially more frequent among patients receiving late-day infusions.
gastric cancer
Survival outcomes
Survival analyses consistently favored early nivolumab administration. Median progression-free survival (PFS) was 2.3 months in the EA group compared with 1.6 months in the LA group, corresponding to a hazard ratio (HR) of 0.65, which was statistically significant. Median overall survival (OS) was also significantly prolonged with early administration (7.6 months vs 3.9 months; HR 0.64).
Importantly, infusion timing remained an independent prognostic factor after multivariable adjustment, with adjusted HRs of 0.70 for PFS and 0.67 for OS, confirming that the observed survival advantage was not solely explained by baseline imbalances.
Sensitivity and robustness analyses
Additional analyses supported the robustness of these findings. When alternative cutoff values were tested, the survival benefit of early administration persisted and became evident once more than approximately 50% of infusions were delivered earlier in the day. Furthermore, in a three-group model stratified by the proportion of early versus late infusions, outcomes followed a clear stepwise pattern: patients treated predominantly in the early hours experienced the most favorable outcomes, those with mixed timing had intermediate results, and patients treated mostly later in the day had the poorest survival.
Safety
- About one-third experienced at least one irAE.
- Most common irAEs: skin toxicity (rash/pruritus), thyroid dysfunction, and transaminase elevation.
- Any-grade irAEs were numerically higher in EA (40.7% vs 29.6%), and skin irAEs were significantly more frequent in EA.
- Grade 3/4 irAEs were similar between groups (~6–7%).
- No treatment-related deaths.
This pattern fits prior observations in ICI practice where the presence of certain irAEs—especially skin toxicities—can correlate with improved outcomes, although causality cannot be inferred here.
Insights
This analysis strengthens a provocative, clinically practical idea: the same drug, given earlier in the day, may be associated with better outcomes—even in a setting as difficult as later-line mGC.
The proposed biological rationale is not about nivolumab’s long plasma half-life; rather it centers on shorter-timescale pharmacodynamics and immune trafficking: if nivolumab distribution to lymphoid tissues and tumor-draining nodes coincides with periods when naïve and memory T cells preferentially localize to lymph nodes, antigen presentation and priming may be more effectively “unblocked,” translating into deeper or more frequent responses. The study also highlights how systemic inflammation markers (like NLR and mGPS) remain powerful prognostic variables in real-world ICI-treated mGC—and timing appeared to retain independent prognostic impact even when accounting for these.
Key Takeaway Messages
In this real-world cohort of third-line+ nivolumab monotherapy for mGC, receiving nivolumab mostly before 14:00 was associated with:
- Higher ORR (17% vs 3%)
- Higher DCR (47% vs 20%)
- Longer PFS (2.3 vs 1.6 months)
- Longer OS (7.6 vs 3.9 months)
The observed association persisted after adjustment in multivariable analyses, confirming infusion timing as an independent factor. Early administration was associated with a higher incidence of immune-related adverse events of any grade, most notably cutaneous toxicities, while no increase in severe (grade 3–4) irAEs was observed. These findings should be interpreted as hypothesis-generating, given the retrospective study design and the potential influence of post-progression treatments on overall survival. Accordingly, prospective, randomized studies are required to validate these observations.
Conclusion
This study suggests that chronotherapy may be an actionable, zero-cost optimization for nivolumab in metastatic gastric cancer—potentially improving efficacy without adding toxicity burden. While the mechanism is not yet defined and confounding is unavoidable in retrospective research, the signal is consistent with chronobiology-informed immunotherapy data from other tumors. The next step is clear: prospective randomized trials and translational work integrating immune-cell dynamics, cortisol rhythms, lymphocyte trafficking, and tissue pharmacokinetics to determine whether “treating earlier” can become a reproducible standard in ICI delivery.
You can read all article here