The DISCUS trial represents one of the most significant steps in rethinking treatment intensity for advanced urothelial carcinoma in the immunotherapy era. With maintenance avelumab firmly established as a global standard of care, the study addressed a fundamental clinical question: how many cycles of platinum-based chemotherapy are truly necessary before starting immunotherapy maintenance?
At the ESMO 2025 Annual Meeting, investigators presented the latest results from this pivotal phase II randomized study, led by Professor Thomas Powles and Dr. Enrique Grande. The findings suggested that reducing chemotherapy duration from six cycles to three does not compromise efficacy and could meaningfully improve patients’ quality of life (Powles et al., 2025).
Scientific Background and Rationale
For decades, standard first-line therapy for advanced or metastatic urothelial carcinoma (mUC) has consisted of four to six cycles of platinum-based chemotherapy, typically gemcitabine plus cisplatin or carboplatin. While these regimens produce measurable responses, cumulative toxicity frequently limits patient adherence. The introduction of avelumab maintenance immunotherapy after chemotherapy response or stable disease reshaped the treatment paradigm, offering durable survival benefits as demonstrated in the JAVELIN Bladder 100 trial (Powles et al., 2020).
However, clinicians continued to administer six chemotherapy cycles largely by convention rather than evidence. Retrospective analyses hinted that extending chemotherapy beyond three or four cycles provided minimal additional survival benefit while exacerbating fatigue, neuropathy, and cytopenias (Necchi et al., 2023). Recognizing this gap, the DISCUS investigators designed the first prospective trial to formally evaluate a shorter induction period before avelumab maintenance.
“The DISCUS trial emerged from a simple but clinically relevant question: do patients with advanced urothelial cancer really benefit from prolonged platinum-based chemotherapy in the era of maintenance immunotherapy?” said Dr. Enrique Grande, co-principal investigator. “We knew from retrospective data that extending chemotherapy beyond three or four cycles added toxicity without clear survival benefit. With avelumab maintenance now standard, we wanted to prospectively test whether shorter induction could preserve efficacy while improving tolerability and patient-reported quality of life.”
Trial Design and Eligibility
The DISCUS trial (NCT06892860) was a multicenter, open-label, phase II randomized study conducted across the United Kingdom, France, and Spain. Between its launch in February 2022 and closure of enrollment in early 2024, the trial accrued 267 patients with locally advanced or metastatic urothelial carcinoma (Powles et al., 2025).
Eligible participants were required to have measurable disease per RECIST v1.1, an ECOG performance status of 0–2, and adequate renal, hepatic, and hematologic function. Both cisplatin- and carboplatin-eligible patients were included, provided they had received no prior systemic therapy for metastatic disease. Patients with autoimmune disorders or contraindications to checkpoint inhibitors were excluded.
Randomization was stratified by platinum agent (cisplatin vs. carboplatin) and presence of liver metastases. Participants were allocated 1:1 to receive either three cycles or six cycles of gemcitabine plus platinum chemotherapy, followed by avelumab maintenance at 800 mg every two weeks for up to two years.
Endpoints and Methods of Assessment
The trial incorporated a dual primary endpoint framework focusing on both patient experience and clinical outcome. These endpoints included:
- Change in global health status/quality of life (GHS/QoL) from baseline to the end of cycle 6, measured using the EORTC QLQ-C30 instrument.
- Overall survival (OS) from randomization.
Secondary objectives comprised progression-free survival (PFS), objective response rate (ORR), safety and tolerability, and additional patient-reported outcomes (PROs).
As Dr. Grande explained, de-escalation trials carry unique operational challenges:
“De-escalation trials are often counterintuitive to both physicians and patients, as they challenge established treatment norms. Convincing investigators and ethics committees that fewer cycles could be equally effective required robust rationale and careful design. Ensuring patient adherence to patient-reported outcome assessments across multiple international sites was also logistically complex.”
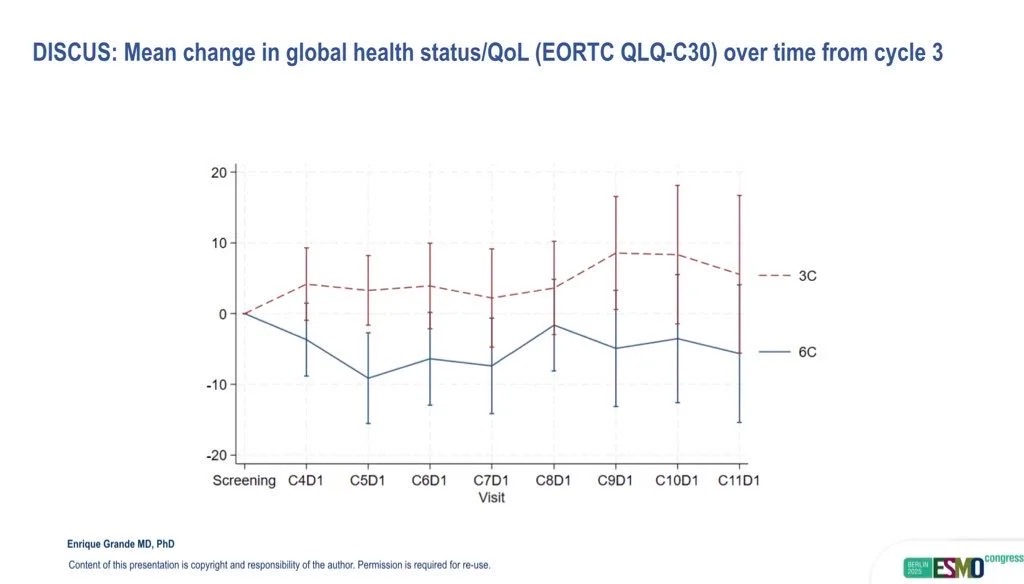
Key Findings and Clinical Outcomes
At the interim analysis presented in late 2025, the DISCUS trial achieved its primary quality-of-life endpoint, demonstrating a statistically and clinically meaningful improvement for patients receiving three cycles of chemotherapy.
Mean change in GHS/QoL score was 0.0 (95% CI –5.9 to +5.2) in the three-cycle arm compared with –8.5 (95% CI –14.1 to –2.9) in the six-cycle arm, resulting in an 8.5-point advantage (95% CI 0.7–16.3; p = 0.016) in favor of shorter therapy (Powles et al., 2025). Importantly, 41% of patients in the three-cycle group reported overall improvement in quality of life compared with 24% in the six-cycle group.
Efficacy outcomes were comparable between arms. Median overall survival reached 18.9 months in both groups (HR = 1.15; 95% CI 0.72–1.86; p = 0.56), while median progression-free survival was 8.0 months in the three-cycle arm versus 9.0 months in the six-cycle arm (HR = 1.05; 95% CI 0.73–1.53). As expected, the trial was not powered to establish non-inferiority.
Tolerability data further supported the de-escalation approach. Fewer grade 3–4 treatment-related adverse events were observed in the three-cycle arm (approximately 12%) compared with the six-cycle arm (around 16%). Discontinuation of therapy due to toxicity occurred in 2% of patients treated with three cycles versus 10% in those treated with six (Powles et al., 2025).
Notably, more patients were able to transition successfully to avelumab maintenance in the three-cycle arm (74%) than in the six-cycle arm (56%). This finding reinforces the hypothesis that early toxicity reduction facilitates continuity of care and greater immunotherapy exposure.
“The comparable progression-free and overall survival, despite fewer chemotherapy cycles, suggests that most therapeutic benefit from platinum occurs early, and that additional cycles may mainly add toxicity,” said Dr. Grande. “Importantly, more patients in the three-cycle arm were able to proceed to maintenance avelumab, which could mitigate any risk of undertreatment. These findings support a shift toward treatment optimization rather than maximal exposure.”
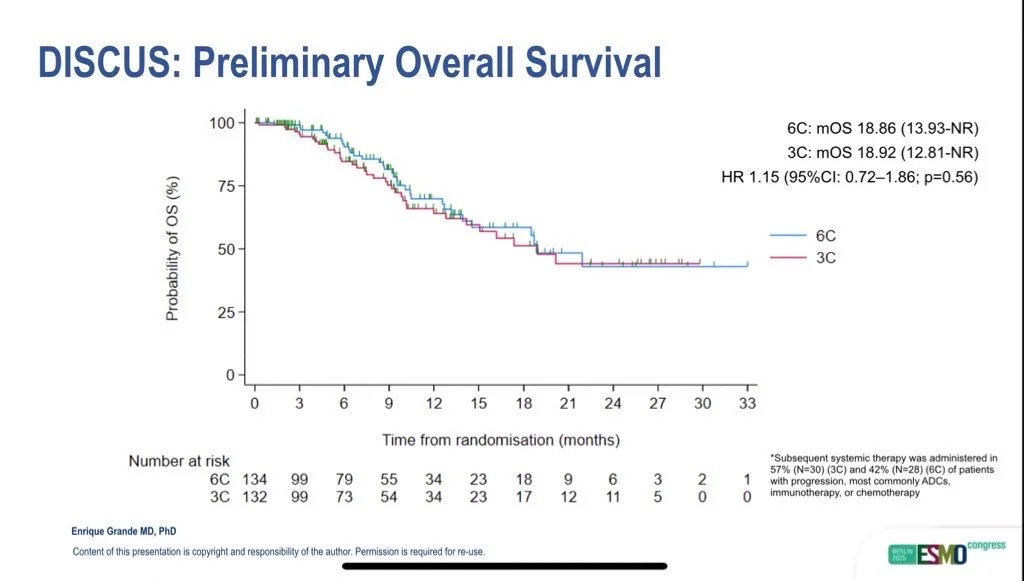
Broader Implications and Future Perspectives
The results of DISCUS align with an evolving philosophy in oncology that prioritizes treatment optimization over intensity. As Dr. Grande emphasized,
“DISCUS demonstrates that less can sometimes be more. In many parts of the world, platinum-based chemotherapy plus maintenance avelumab remains a standard option. For these patients, limiting chemotherapy to three cycles can improve quality of life without compromising outcomes.”
Beyond urothelial carcinoma, the trial illustrates that prospective de-escalation studies—long considered logistically and ethically challenging—can be both feasible and scientifically informative. Its success opens doors for similar designs in lung, head and neck, and gynecologic malignancies, particularly in settings where maintenance or targeted agents have transformed treatment duration considerations.
The next steps for DISCUS include continued follow-up to mature overall survival data, planned exploratory biomarker analyses, and health-economic modeling to assess cost-effectiveness. Several cooperative groups are already designing follow-up phase III studies to validate the approach in broader, real-world populations.
Conclusion
The DISCUS trial provides convincing prospective evidence that three cycles of platinum-based chemotherapy before avelumab maintenance can maintain oncologic efficacy while improving quality of life and reducing treatment burden for patients with advanced urothelial carcinoma. Its implications extend beyond bladder cancer, signaling a broader paradigm shift toward personalized treatment duration.
“These findings support a shift toward treatment optimization rather than maximal exposure,” concluded Dr. Grande. For our patients, especially those facing the dual challenge of cancer and cumulative toxicity, DISCUS offers a data-driven path to care that is both effective and humane.”
Read About Full Article Here
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


