Platinum-based chemotherapy followed by maintenance avelumab remains a key first-line approach for patients with advanced or metastatic urothelial cancer (mUC), especially where the enfortumab vedotin plus pembrolizumab (EVP)regimen is not yet accessible. Traditionally, six cycles of chemotherapy are administered before initiating maintenance immunotherapy. However, this practice lacks strong evidence and may compromise quality of life (QoL) while exposing patients to cumulative toxicity.
The DISCUS trial was initiated to test whether a shortened chemotherapy duration (three cycles) could preserve efficacy while improving patient-reported outcomes, addressing an important question in real-world global oncology practice.
Study Design and Methods
The phase II, randomized, open-label DISCUS trial enrolled 267 patients with advanced or metastatic urothelial carcinoma across sites in the UK, France, and Spain. Patients were randomized 1:1 to:
- 3C Arm: Three cycles of gemcitabine/cisplatin or gemcitabine/carboplatin followed by avelumab (800 mg Q2W)
- 6C Arm: Six cycles of the same chemotherapy regimen followed by avelumab
Key co-primary endpoints were:
- Change in Global Health Status/Quality of Life (GHS/QoL) score (EORTC QLQ-C30) from baseline to cycle 6 completion.
- Overall survival (OS) measured from randomization.
Secondary endpoints included progression-free survival (PFS), overall response rate (ORR), and safety.
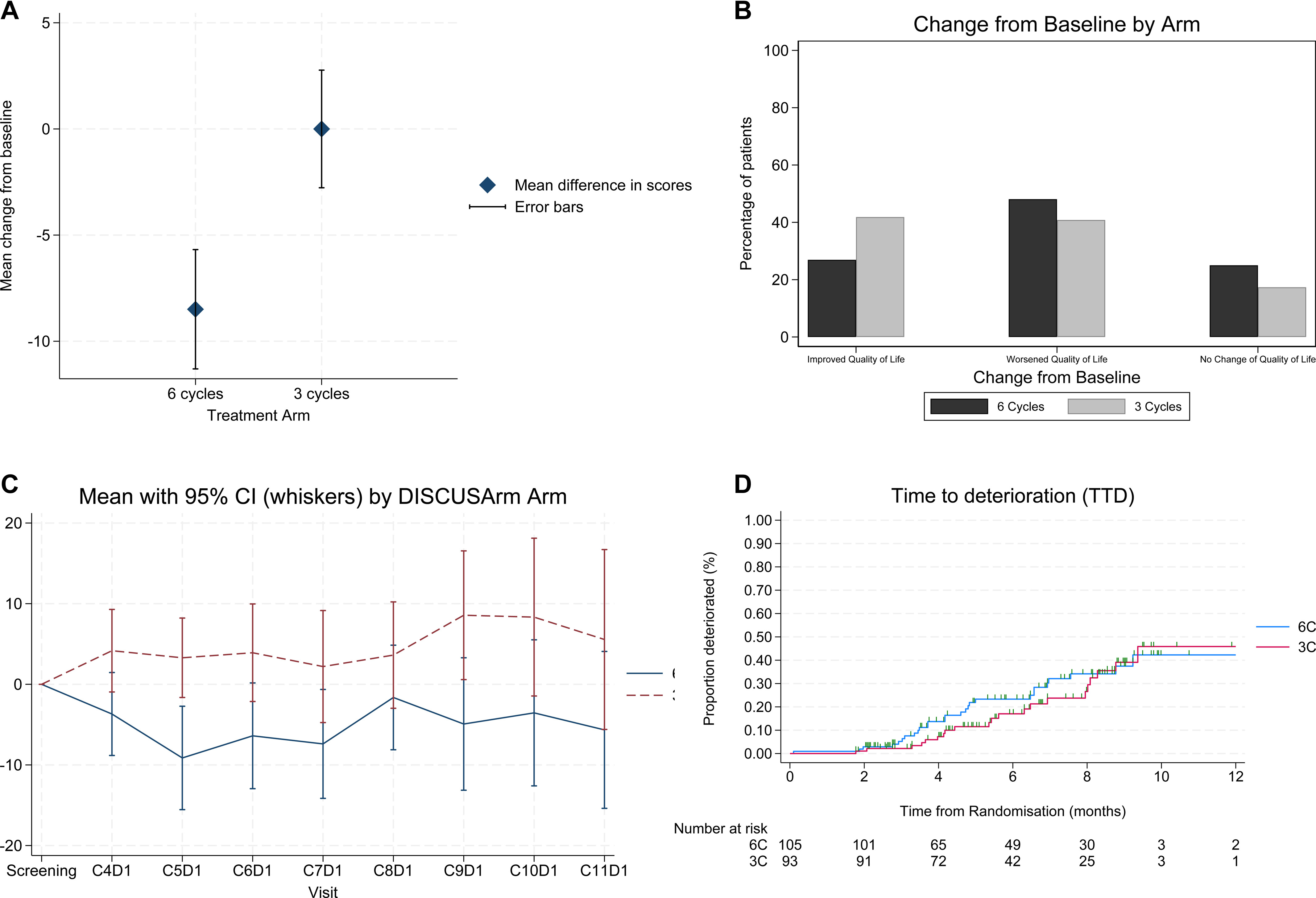
Results
Quality of Life
QoL outcomes favored the three-cycle arm:
- Mean GHS/QoL change: 0.0 (95% CI –5.9, 5.2) in 3C vs –8.5 (95% CI –14.1, –2.9) in 6C.
- Between-arm difference: +8.5 points (95% CI 0.7–16.3; p = 0.016).
- Improvement in PROs observed in 41% (3C) vs 24% (6C) of patients.
Although this did not reach the 10-point threshold for a “clinically meaningful difference,” the 8.5-point improvement lies within the minimally important difference (MID) range of 5–10 points, indicating real-world relevance.
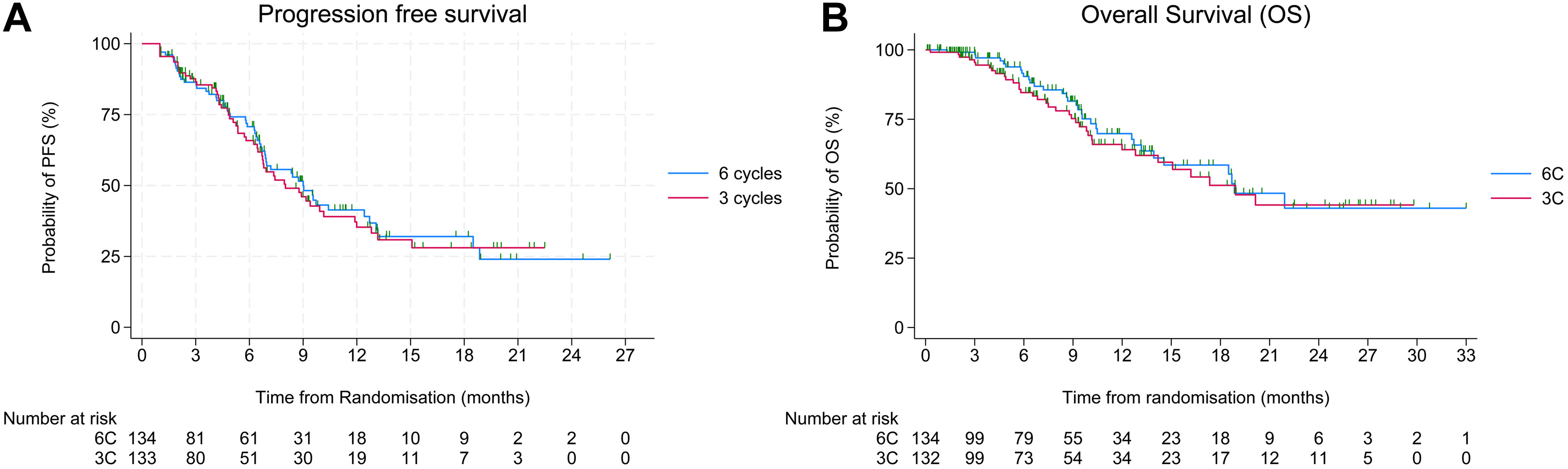
Efficacy
- Median OS: 18.9 months in both arms (HR = 1.15; 95% CI 0.72–1.86; p = 0.56).
- Median PFS: 8.0 months (3C) vs 9.0 months (6C) (HR = 1.05; p = 0.79).
- ORR: 24% (3C) vs 27% (6C).
- Avelumab exposure: 74% of 3C patients received maintenance therapy vs 56% in 6C.
The study was underpowered for non-inferiority, and longer follow-up is ongoing for the final OS analysis.
Safety
Treatment-related adverse events (TRAEs) of any grade occurred in 86% (3C) vs 90% (6C), with grade 3–4 TRAEs in 40% vs 54%, respectively.
Common TRAEs included anaemia, neutropenia, nausea, and fatigue, with slightly higher rates of hematologic toxicity in the 6C arm.
Serious AEs occurred in 35% (3C) vs 37% (6C).
Interpretation
The DISCUS trial demonstrates that reducing platinum-based chemotherapy from six to three cycles before maintenance avelumab leads to significantly better short-term QoL without compromising early survival outcomes. Although OS non-inferiority was not established, the feasibility and patient-centered design of this trial mark an important step toward de-escalation strategies in metastatic urothelial cancer.
These findings suggest that shorter chemotherapy induction may be a reasonable approach for patients experiencing toxicity or limited tolerance, especially in cisplatin-eligible subgroups, where QoL benefits were most evident.
Final OS results from the ongoing analysis will further clarify whether a reduced chemotherapy duration can safely replace the standard six-cycle approach.
Key Takeaways
- Three cycles of platinum chemotherapy before avelumab maintenance improved quality of life vs six cycles.
- No significant difference in overall or progression-free survival at interim analysis.
- Lower rates of grade 3–4 toxicity observed in the 3C arm.
- Higher completion and avelumab initiation rates in the 3C group.
- The trial supports feasibility of shorter systemic therapy duration in mUC, emphasizing patient-reported outcomes as critical endpoints.
Clinical Significance
While EVP (enfortumab vedotin + pembrolizumab) is emerging as the first-line global standard, many regions still rely on platinum-based regimens. The DISCUS results highlight a pragmatic, patient-friendly alternative that optimizes QoL without compromising early efficacy—especially relevant in settings where access to newer agents is limited or where treatment tolerability dictates therapy choice.
Future trials will be needed to test shortened induction regimens with maintenance immunotherapy or ADCs, potentially redefining treatment duration standards across solid tumors.
You Can Read Full Article Here


