The DISCUS trial (NCT06892860) is redefining the standard approach to chemotherapy duration in advanced urothelial carcinoma (aUC). For more than a decade, six cycles of platinum-based chemotherapy followed by maintenance avelumab has served as the global standard of care. While this regimen improves overall survival, it also results in cumulative toxicity, treatment fatigue, and a significant decline in quality of life (QoL).
As maintenance immunotherapy has become an integral part of managing aUC, oncologists have increasingly questioned whether reducing the number of chemotherapy cycles could achieve comparable outcomes with fewer side effects. The DISCUS trial, conducted by Queen Mary University of London and supported by Merck, was designed to answer this critical clinical question.
By comparing three cycles versus six cycles of first-line platinum-based chemotherapy prior to maintenance avelumab, the DISCUS trial sought to determine whether a shorter chemotherapy regimen could preserve efficacy while improving tolerability and patient well-being. The results presented at ESMO 2025 provide valuable evidence that fewer chemotherapy cycles may deliver similar survival outcomes with significantly better QoL — marking a step forward in patient-centered care for urothelial cancer.
Method
In this randomized, open-label phase 3 trial, 267 patients with previously untreated advanced urothelial carcinoma were assigned equally to receive either three (3C) or six (6C) cycles of gemcitabine combined with cisplatin or carboplatin, followed by maintenance avelumab.
The dual primary endpoints were overall survival (OS) and patient-reported quality of life, measured using the EORTC QLQ-C30 Global Health Status/Quality of Life (GHS/QoL) scale. Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), and safety.
The trial sought to test whether reducing chemotherapy exposure could maintain survival outcomes while reducing treatment-related toxicity and improving patient experience.
Results
The data cutoff was August 25, 2025. Of the 267 randomized patients, 133 were assigned to the 3C arm and 134 to the 6C arm. Baseline demographics were balanced between the two groups, and approximately 42% received cisplatin-based therapy.
Most patients were able to complete their allocated treatment: 78% in the 3C group and 40% in the 6C group finished all planned cycles. Importantly, more patients in the 3C arm proceeded to maintenance avelumab (74% vs 56%), indicating better tolerance and continuity of care.
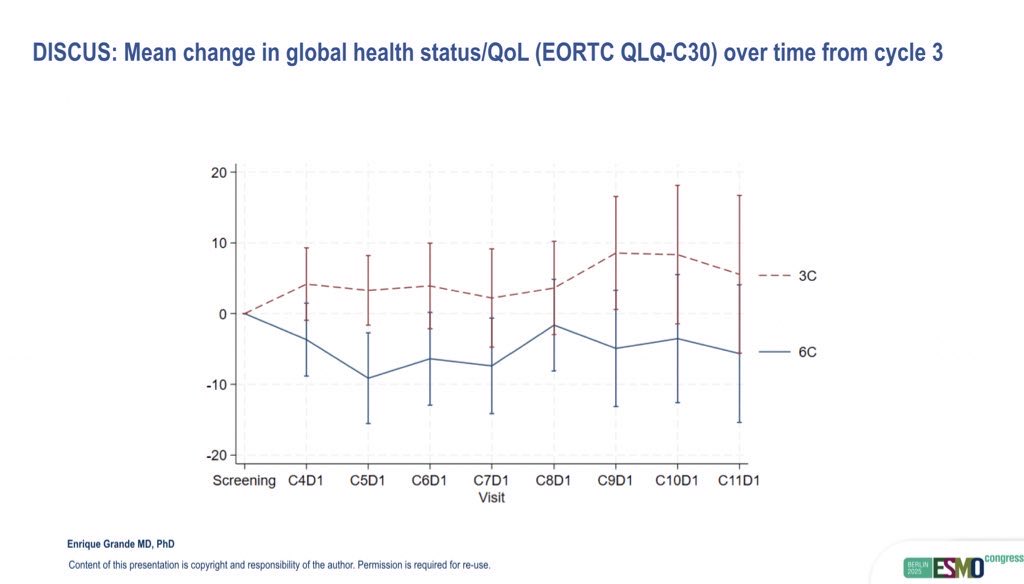
Quality of life outcomes strongly favored the shorter regimen. The mean change in QoL from baseline to cycle 6 was 0.0 (95% CI: –5.9 to 5.2) in the 3C group, compared to –8.5 (95% CI: –14.1 to –2.9) in the 6C group, representing a clinically meaningful +8.5-point improvement (95% CI: 0.7–16.3; p=0.016). Improvement in overall patient-reported outcomes was observed in 41% of patients in the 3C arm, versus 24% in the 6C arm.
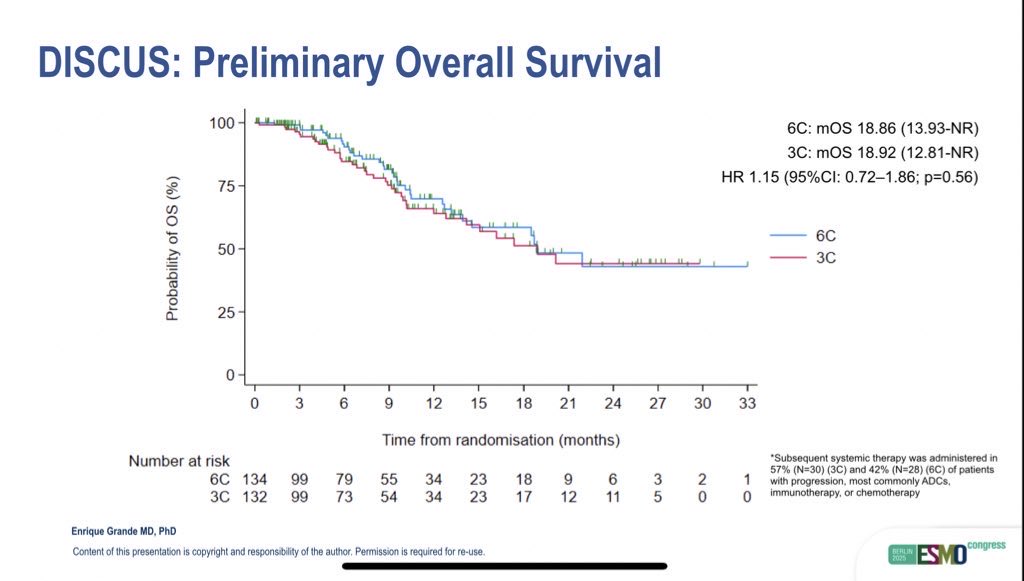
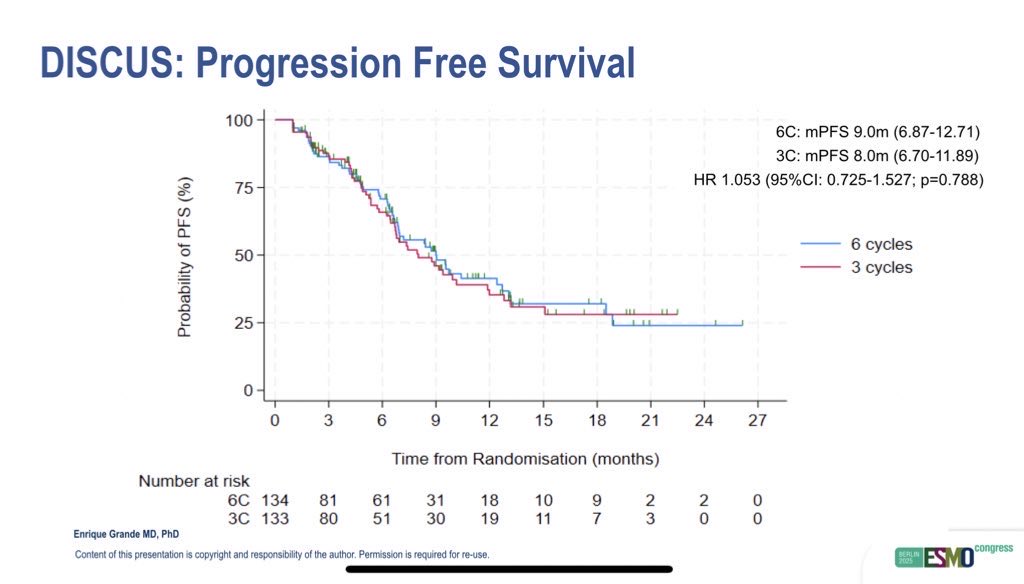
Despite receiving fewer chemotherapy cycles, efficacy outcomes were remarkably similar between groups. The objective response rate (ORR) was 24% in the 3C arm and 27% in the 6C arm. Median PFS was 8.0 months (95% CI: 6.7–11.9) versus 9.0 months (95% CI: 6.9–12.7), and median OS was identical at 18.9 months in both groups (HR 1.15, 95% CI: 0.72–1.86; p=0.56).
Toxicity was lower with the shorter chemotherapy regimen. Grade 3–4 treatment-related adverse events occurred in 11.9% of patients in the 3C arm compared with 15.7% in the 6C arm. Most adverse events were hematologic or gastrointestinal and were manageable with supportive care. No new safety concerns emerged with maintenance avelumab.
Long-Term Follow-Up and Analytical Considerations
The interim analysis suggests that three cycles of platinum-based chemotherapy followed by avelumab offers comparable disease control and survival outcomes to six cycles, with superior tolerability and improved patient experience. By allowing patients to transition to maintenance therapy sooner, the three-cycle strategy reduces treatment fatigue and maintains QoL without sacrificing efficacy.
Ongoing follow-up will assess long-term survival, but current results already support the concept of minimizing chemotherapy exposure in eligible patients. These findings could influence clinical practice by prioritizing patient-centered treatment plans that preserve both survival and quality of life.
Read Full Abstract on ESMO Congress 2025 Website
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


