CONgRAtS trial (2687MO), presented by Dr. Florent Peyraud at the ESMO 2025 Congress, is the first randomized study to investigate nivolumab with or without relatlimab in patients with tertiary lymphoid structure–positive (TLS+) soft-tissue sarcomas (STS). Conducted across multiple French sarcoma centers, the trial evaluated whether dual PD-1 and LAG-3 blockade could enhance clinical outcomes beyond PD-1 inhibition alone in this biomarker-defined population.
Background
Immune checkpoint inhibitors have shown limited benefit in unselected STS, largely due to the heterogeneous and often immunologically “cold” tumor microenvironment. The presence of tertiary lymphoid structures (TLS) has emerged as a predictive biomarker of response to PD-1 blockade, as first validated in the PEMBROSARC trial (Nat Med 2022). However, not all TLS-positive patients respond to PD-1 monotherapy, suggesting additional inhibitory pathways at play.
LAG-3, frequently co-expressed with PD-1 on exhausted intratumoral T cells, represents one such pathway. The CONgRAtS trial was designed to test whether dual PD-1/LAG-3 blockade with nivolumab + relatlimab could further improve antitumor immunity in patients with TLS+ advanced STS.
Methods
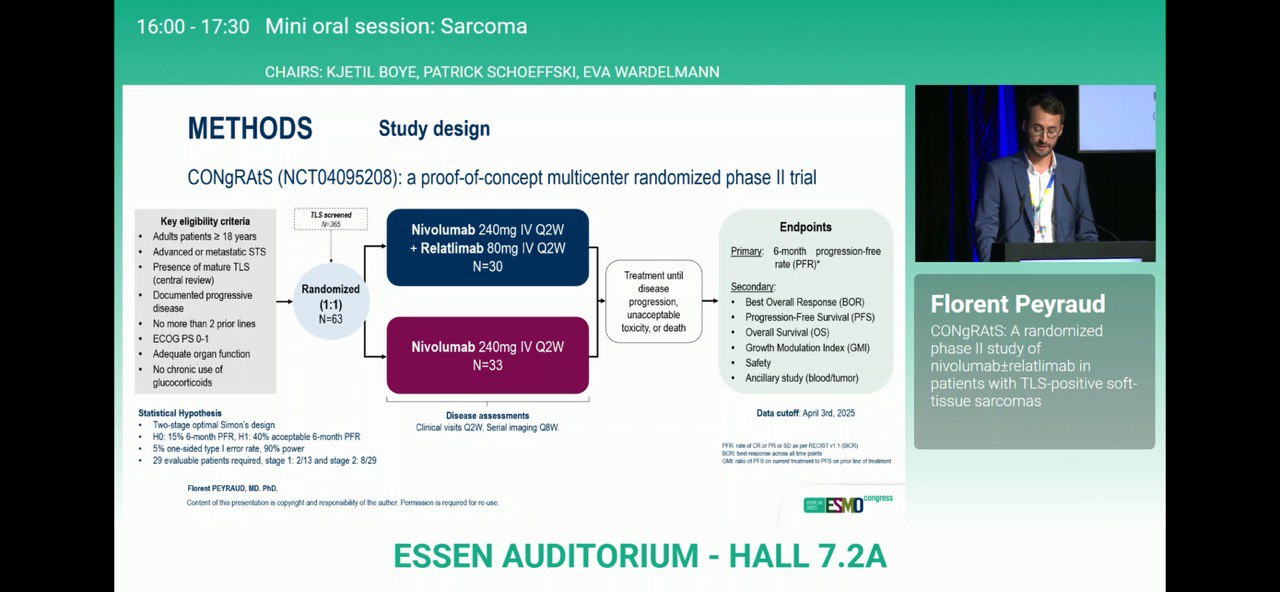
This multicentre, open-label, randomized Phase II study enrolled patients with advanced or metastatic TLS-positive STS, confirmed through central pathology review. Participants were randomized 1:1 to:
- Arm A: nivolumab + relatlimab
- Arm B: nivolumab monotherapy
The primary endpoint was 6-month progression-free rate (PFR) per RECIST 1.1.Secondary endpoints included progression-free survival (PFS), overall survival (OS), and safety.Tumor biopsies and blood samples were analyzed using spatial transcriptomics (Visium HD, Xenium) and proteomics (Olink) to explore immune correlates of response.
Results
Of 332 patients screened, 68 (20.5%) were confirmed TLS-positive and randomized (34 per arm).
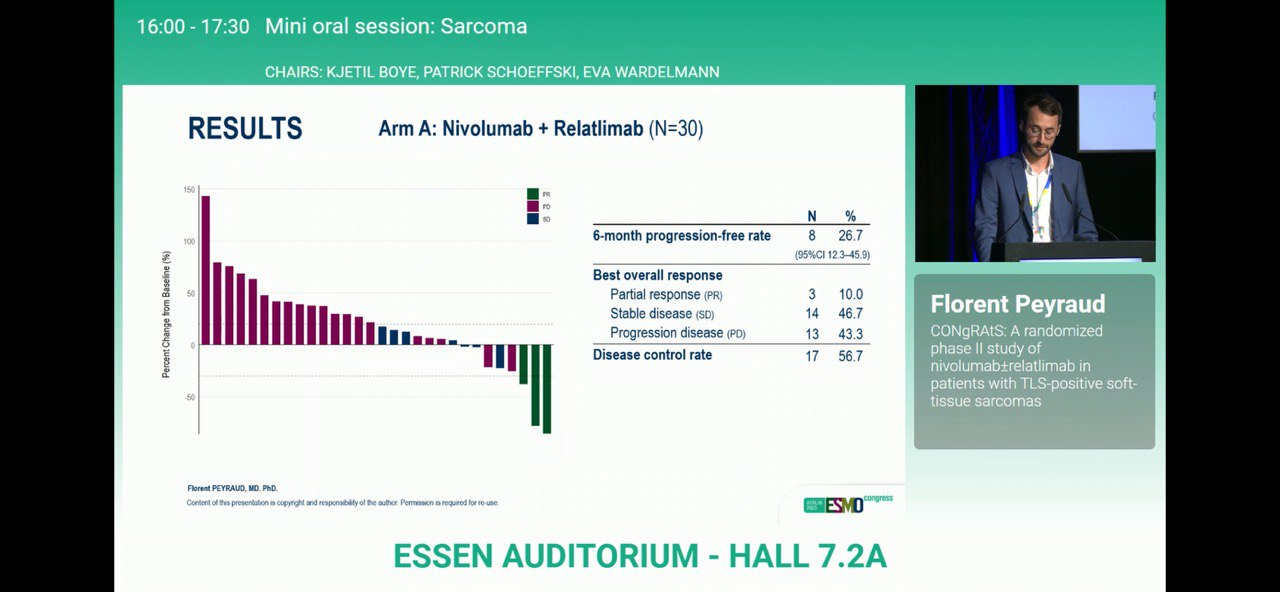
- 6-month PFR: 26.7% (95% CI 12.3–45.9) with nivolumab + relatlimab vs 39.4% (95% CI 22.9–57.9) with nivolumab alone.
- Median PFS: 3.7 months (95% CI 1.8–5.8) vs 5.2 months (95% CI 2.8–9.4).
- Median OS: 12.6 months (95% CI 9.3–19.8) vs 29.0 months (95% CI 12.7–NR).
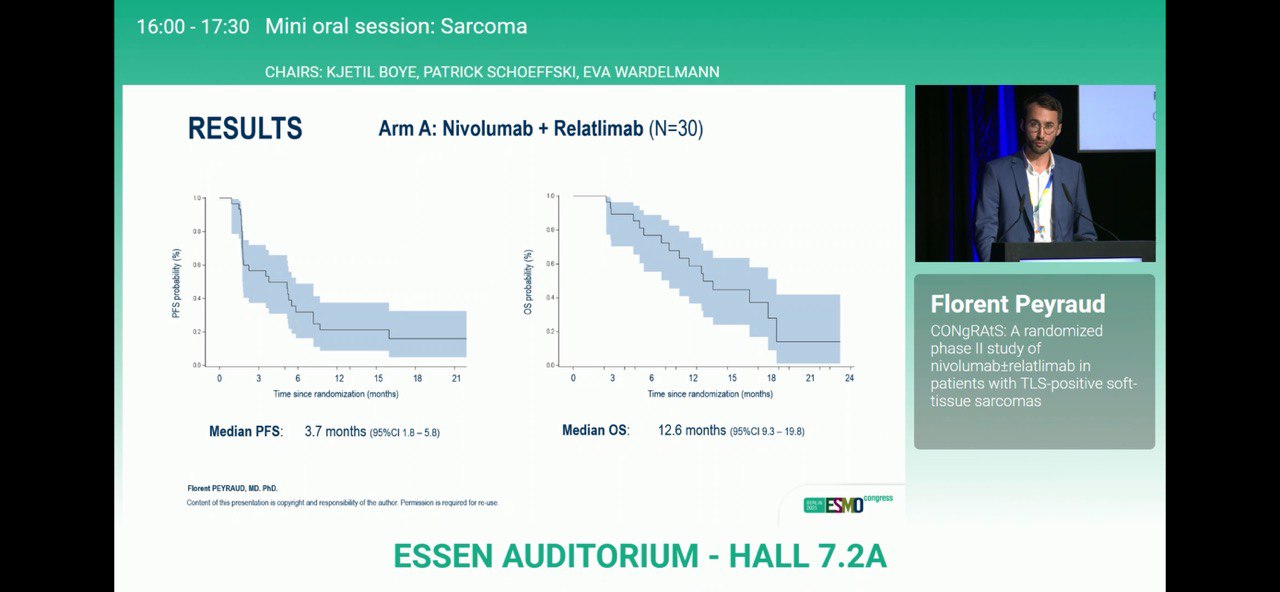
Both arms met the predefined primary efficacy threshold and exceeded historical benchmarks observed in unselected STS populations.
Grade ≥3 treatment-related adverse events occurred in 32.4% of patients on the combination and 29.4% on nivolumab alone, with no unexpected toxicities
Preliminary translational analyses revealed immune architectural differences between responders and non-responders, including variations in TLS maturation, B-cell/plasma cell enrichment, and chemokine profiles. Further integrative immune profiling is ongoing.
Key Insights
The CONgRAtS trial represents the first prospective validation of TLS as a predictive biomarker in sarcoma immunotherapy. While the addition of LAG-3 inhibition did not enhance clinical efficacy beyond PD-1 blockade, both treatment arms demonstrated meaningful activity compared with historical outcomes. These findings underscore the clinical relevance of TLS-guided patient selection and highlight the heterogeneity of immune responses within TLS+ tumors.
Clinical Significance
CONgRAtS establishes TLS as a clinically actionable biomarker for immunotherapy in STS, paving the way for biomarker-driven trial designs in this rare tumor group. Although relatlimab did not improve efficacy, ongoing translational work may clarify mechanisms of resistance and guide the development of next-generation immunotherapy combinations tailored to sarcoma immune microenvironments.

You can read the full abstract here.


