This article explores how artificial intelligence is transforming every aspect of cancer care—from improving early detection and diagnostic accuracy to personalizing treatment, streamlining clinical trials, and enhancing patient support. You’ll learn about real-world AI applications, current benefits and limitations, and emerging trends shaping the future of oncology.
What Is a Clinical Trial?
A clinical trial is a scientifically rigorous study conducted in human participants to evaluate the safety, efficacy, and optimal use of new medical interventions—such as drugs, biologics, devices, or treatment protocols—under carefully controlled conditions. These trials follow a predefined protocol that outlines objectives, eligibility criteria, treatment plans, and methods for monitoring outcomes and adverse events (U.S. Food and Drug Administration, 2020).
Clinical trials are typically organized into sequential phases. Phase I involves a small number of healthy volunteers or patients to determine safe dosage ranges and identify potential side effects. Phase II expands to larger patient cohorts to gather preliminary data on efficacy and further assess safety. Phase III compares the new intervention against the current standard of care in hundreds or thousands of patients to confirm therapeutic benefit, evaluate risk–benefit balance, and support regulatory approval. After approval, Phase IV (post-marketing) studies monitor long-term safety and effectiveness in broader, real-world populations (Friedman et al., 2015).
By adhering to ethical guidelines—such as obtaining informed consent from all participants and oversight by institutional review boards—clinical trials ensure that new treatments are tested responsibly and transparently, ultimately generating the high-quality evidence needed to advance medical practice and improve patient outcomes (World Health Organization, 2011).
Why Clinical Trials Matter in Cancer Research
Clinical trials are the cornerstone of progress in cancer research, providing the rigorous evidence needed to introduce new therapies safely and effectively. By comparing novel treatments against existing standards in controlled settings, randomized controlled trials establish the true benefit and risk profile of interventions, guiding regulatory approval and clinical practice (U.S. National Cancer Institute, 2024). In oncology, this process has led to paradigm-shifting advances such as targeted therapies and immunotherapies, which dramatically improve survival and quality of life for many patients.
Beyond evaluating drug efficacy, clinical trials play a critical role in identifying predictive biomarkers and refining patient selection, ushering in the era of precision oncology (Hay et al., 2014). Molecularly guided trials match treatments to each tumor’s genetic profile, maximizing benefit and minimizing unnecessary toxicity. Trials also offer patients early access to cutting-edge therapies before they are widely available, which can be particularly impactful for those with advanced or refractory disease.
Furthermore, oncology trials generate real-world evidence on long-term outcomes, safety in diverse populations, and optimal treatment sequencing. They inform clinical guidelines and practice standards worldwide, ensuring that care evolves in step with the latest scientific discoveries. Ultimately, without cancer clinical trials, innovations would remain confined to the laboratory, and patient outcomes would stagnate. These studies embody both the scientific rigor and ethical commitment required to translate laboratory breakthroughs into lifesaving therapies (World Health Organization, 2011).
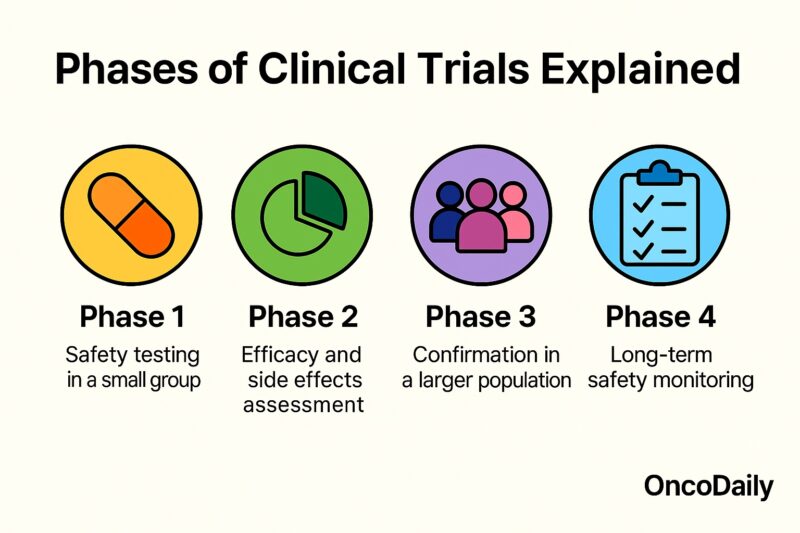
Phases of Clinical Trials Explained
Clinical trials progress through sequential phases designed to rigorously evaluate new interventions in humans. Each phase has distinct objectives, patient populations, and study designs to ensure safety and efficacy before a therapy becomes standard care.
Phase I trials are the first step in testing a new treatment in humans. Typically involving 20–80 healthy volunteers or patients, these studies focus on determining the safest dose range, identifying side effects, and characterizing pharmacokinetics (how the drug is absorbed, distributed, metabolized, and excreted) (U.S. Food and Drug Administration, 2020).
Phase II trials expand to several dozen to a few hundred patients with the target disease. The primary goal is to assess preliminary efficacy—whether the intervention produces the desired therapeutic effect—and continue safety evaluations. Phase II results inform optimal dosing regimens and guide the design of larger trials (Friedman et al., 2015).
Phase III trials involve hundreds to thousands of patients and compare the new intervention against the current standard of care, often using randomized controlled designs. These pivotal studies confirm efficacy, monitor adverse reactions in diverse populations, and collect data to support regulatory approval. Successful Phase III results form the basis for submission to health authorities such as the FDA or EMA (World Health Organization, 2011).
Phase IV (post-marketing) studies occur after approval and assess long-term safety, effectiveness in real-world settings, and rare or delayed adverse events. Phase IV trials may also explore new indications, patient subgroups, or drug combinations, ensuring continuous evaluation of benefit–risk profiles over time (Friedman et al., 2015).
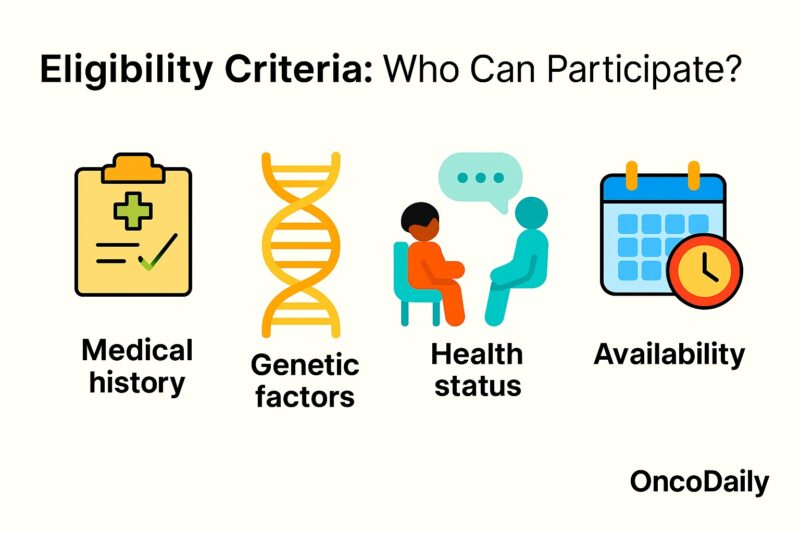
Eligibility Criteria: Who Can Participate?
Eligibility for a clinical trial is determined by predefined inclusion and exclusion criteria designed to select participants most likely to benefit from—and least likely to be harmed by—the investigational treatment. Common inclusion criteria in oncology trials include confirmed cancer diagnosis of a specific type and stage, measurable disease by RECIST or other standardized criteria, acceptable organ function (liver, kidney, bone marrow), and performance status (e.g., ECOG 0–1) (Doroshow et al., 2016). Many trials also specify prior treatment history, such as the number and type of prior chemotherapy or biologic therapies, and may require certain genetic or biomarker profiles (e.g., EGFR mutation–positive lung cancer) to enroll patients most likely to respond (Dienstmann et al., 2019).
Conversely, exclusion criteria protect patient safety by omitting individuals at higher risk of severe toxicity or complications. These commonly exclude patients with significant comorbidities (e.g., uncontrolled heart disease, active infections), inadequate organ function, or concomitant use of contraindicated medications. Trials may also exclude pregnant or breastfeeding women, minors, and those unable to provide informed consent (Williams et al., 2010). By carefully balancing inclusion and exclusion factors, trial designers aim to create a study population that can safely demonstrate the investigational therapy’s true efficacy and safety profile.
How to Find and Enroll in a Trial
Locating and joining a suitable clinical trial can be a transformative opportunity for patients and a crucial step in advancing cancer research. To begin, patients or healthcare providers can search centralized registries such as ClinicalTrials.gov—a service of the U.S. National Library of Medicine—which lists detailed information on thousands of ongoing trials worldwide, including eligibility criteria, study locations, and contact information (Dunlop et al., 2014). The National Cancer Institute’s Cancer Information Service also maintains a searchable database and offers guidance on interpreting trial protocols and finding trials that match a patient’s disease type, stage, and prior treatments (NCI, 2023).
Once a promising trial is identified, the next step is to review the inclusion and exclusion criteria carefully. These criteria define the patient population the trial is designed to study—factors like cancer subtype, genetic markers, prior therapies, and organ function are common considerations (Ford et al., 2008). Patients should discuss these criteria with their oncologist to confirm eligibility and to understand any potential risks or benefits.
After confirming eligibility, interested patients or their physicians contact the trial site’s research team—details for which are provided in the registry listing. The research team will then conduct a prescreening assessment, which may include medical record review, laboratory tests, and additional imaging or biomarker studies. If pre–screening is successful, the patient is invited to an informed consent discussion, in which study personnel review the trial’s purpose, procedures, potential side effects, and patient rights. Only after signed informed consent can a patient be formally enrolled and begin trial participation, under the oversight of an institutional review board (IRB) to ensure ethical conduct (World Health Organization, 2011).
By leveraging these structured resources and following this stepwise process—searching reputable registries, evaluating trial criteria with their care team, and engaging with research staff—patients can access cutting-edge therapies and contribute to the development of tomorrow’s standard of care.
Understanding Informed Consent
Informed consent is a foundational ethical requirement in clinical trials, ensuring that participants voluntarily agree to enroll with full knowledge of the study’s nature, potential risks, and benefits. The process begins with a clear explanation of the trial’s purpose, procedures, duration, and any experimental aspects. Participants must be informed of alternative treatments and their right to withdraw at any time without penalty or loss of standard care (World Medical Association, 2013).
Key elements of informed consent include:
- Disclosure: Investigators provide comprehensive information about the intervention, study design, possible side effects, and unknowns.
- Comprehension: Consent materials and discussions are tailored to the participant’s language, culture, and education level to ensure understanding.
- Competence: Only individuals capable of making informed decisions—those without impairing cognitive conditions—are enrolled.
- Voluntariness: Participation must be entirely voluntary, free from coercion or undue influence, with clear reassurance that declining or withdrawing will not affect regular medical care (ICH, 2016).
Documentation typically involves a written consent form signed by the participant (or legally authorized representative) and the investigator. This form is reviewed and approved by an Institutional Review Board (IRB) or independent ethics committee to ensure ethical compliance and regulatory standards are met (U.S. Department of Health and Human Services, 2017).
By rigorously adhering to informed consent principles, clinical trials honor patient autonomy and safeguard the integrity of the research process.
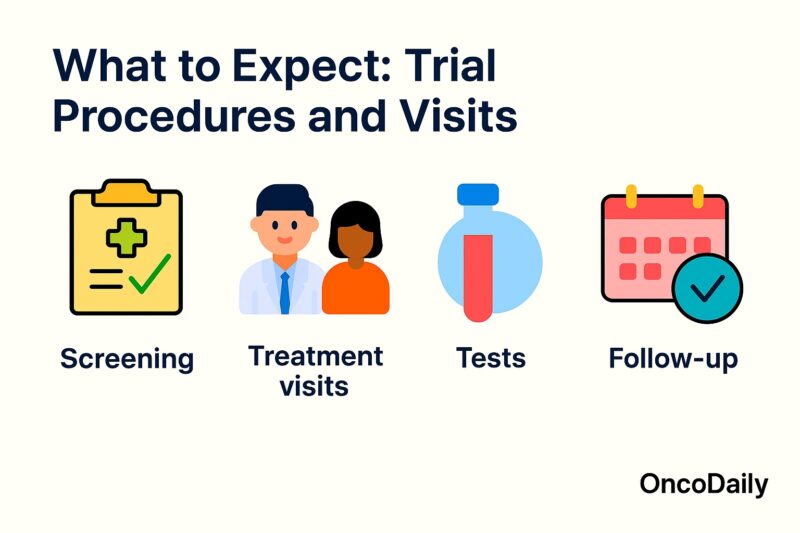
What to Expect: Trial Procedures and Visits
When you enroll in a cancer clinical trial, you become an active partner in your care, and the study team will guide you through a series of scheduled visits and procedures designed to evaluate the investigational treatment’s safety and effectiveness. While specific requirements vary by protocol, most oncology trials include the following elements:
Screening and Baseline Assessment
Before treatment begins, you will undergo a screening visit to confirm eligibility. This typically includes a review of your medical history, physical examination, performance status assessment (e.g., ECOG), blood tests, imaging studies (CT, MRI, PET), and any necessary biopsies or cardiac evaluations. These baseline measurements establish your health status prior to receiving the study intervention and allow for accurate comparisons over time (Friedman et al., 2015).
Treatment Visits
Once enrolled, you will attend treatment visits at intervals defined by the study protocol—often every 1 to 3 weeks. During these visits, you may receive the investigational drug (oral, IV infusion, or injection) and standard supportive medications. The research team will monitor your vital signs, administer protocol-specific assessments, and manage any side effects. Blood draws are common to check safety labs (e.g., complete blood count, liver and kidney function) and, in some trials, to collect pharmacokinetic or biomarker data (U.S. Food and Drug Administration, 2020).
Imaging and Response Evaluation
At predetermined time points (e.g., every 8–12 weeks), you will undergo radiologic imaging—such as CT, MRI, or PET scans—to evaluate tumor response according to standardized criteria (RECIST). These scans help determine whether the cancer is shrinking, stable, or progressing, guiding decisions about continuing, modifying, or stopping the study treatment (Eisenhauer et al., 2009).
Safety and Adverse Event Monitoring
Throughout the trial, the study team will closely monitor for adverse events (AEs), documenting their type, severity (graded per CTCAE), onset, and possible relation to the investigational agent. Serious adverse events (SAEs) are reported immediately to regulatory authorities and IRBs. You may also be asked to complete symptom questionnaires or quality-of-life instruments to capture patient-reported outcomes (Basch et al., 2006).
Follow-Up Visits
After completing the treatment phase—or if the study drug is discontinued due to progression or toxicity—you will enter a follow-up period. These visits may occur monthly, quarterly, or at longer intervals to assess long-term safety, survival, and any late effects of therapy. Follow-up ensures that both you and future patients benefit from a complete understanding of the treatment’s impact over time (Friedman et al., 2015).
By understanding these trial procedures and visit schedules, you can prepare for the commitment, communicate effectively with your care team, and actively participate in the clinical research that drives advances in cancer treatment.
Potential Benefits and Risks
Participation in a cancer clinical trial offers several important benefits. Patients may gain early access to cutting-edge therapies before they become widely available, which can be especially valuable for those whose disease has not responded to standard treatments (U.S. National Cancer Institute, 2024). Trials also provide enhanced medical monitoring, with frequent assessments of safety and response that often exceed the intensity of routine care. By contributing to research, participants help advance scientific knowledge and may improve outcomes for future patients with similar cancers (Ferns et al., 2018).
However, clinical trials also carry inherent risks. New therapies under investigation may have unforeseen side effects or toxicities that only become apparent in larger patient populations, and the balance of benefit versus harm is not yet fully established (U.S. Food and Drug Administration, 2020). Some trials include placebo or control arms, which means a participant might not receive the experimental treatment. Additionally, trial protocols often require extra visits, procedures, and tests, imposing a time and travel burden on patients and caregivers. In rare cases, investigational agents have been associated with serious adverse events that necessitate early discontinuation of therapy (Denicoff et al., 2013).
By weighing these potential benefits and risks in consultation with their oncology team, patients can make informed decisions about trial participation. Ethical oversight by institutional review boards and rigorous informed-consent processes are in place to protect participants and ensure that trials proceed with the highest standards of patient safety (World Medical Association, 2013).
Safety Monitoring and Patient Rights
Clinical trials in oncology are conducted under stringent safety monitoring frameworks to protect participants and uphold ethical standards. Safety monitoring begins with predefined adverse event (AE) reporting procedures that classify and grade side effects according to the Common Terminology Criteria for Adverse Events (CTCAE). Trial investigators record every AE—ranging from mild laboratory abnormalities to life-threatening reactions—and report serious adverse events (SAEs) immediately to the study sponsor, ethics committees, and regulatory authorities (U.S. Food and Drug Administration, 2020).
To ensure impartial oversight, many trials employ an independent Data Safety Monitoring Board (DSMB). The DSMB periodically reviews unblinded safety data and has the authority to recommend protocol modifications or trial suspension if unexpected risks emerge (Ellenberg et al., 2003). In addition, site investigators perform regular monitoring visits and laboratory assessments—such as blood counts, liver and kidney function tests, and electrocardiograms—at scheduled intervals or whenever clinically indicated.
Patient rights are equally safeguarded. Before enrollment, participants undergo a thorough informed consent process, where they learn about potential risks, benefits, alternative treatments, and their right to withdraw at any time without penalty (World Medical Association, 2013). Consent forms are reviewed and approved by independent Institutional Review Boards (IRBs) or ethics committees, ensuring the study’s scientific validity and ethical compliance.
Throughout the trial, participants retain the right to privacy and data confidentiality. Personal health information is de-identified and stored securely, in compliance with regulations such as the Health Insurance Portability and Accountability Act (HIPAA) in the U.S. or the General Data Protection Regulation (GDPR) in Europe. Participants also receive clear communication about any new safety findings or changes in trial procedures that may affect their willingness to continue.
By combining rigorous safety monitoring with robust protections for patient autonomy and privacy, oncology clinical trials maintain the highest standards of ethical conduct, ensuring that new therapies are tested responsibly and that participants’ well-being remains the foremost priority.
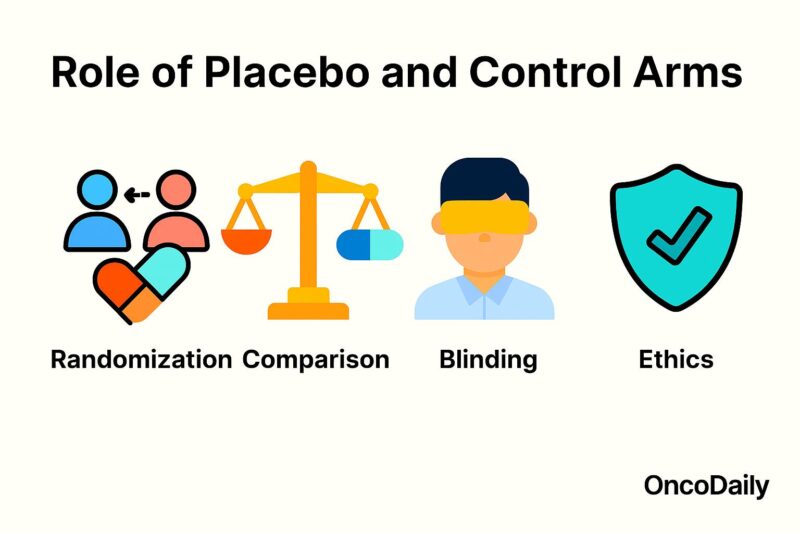
Role of Placebo and Control Arms
Control arms—including placebo groups—serve as indispensable comparators in oncology clinical trials, enabling researchers to discern the true effects of an investigational therapy from natural disease progression and placebo responses. By randomly assigning participants either to the experimental treatment or to a control regimen, trials minimize selection bias and confounding factors, ensuring that observed differences in outcomes reflect the treatment itself rather than patient or disease characteristics (Fayers & Machin, 2016).
In many cancer studies, the control arm consists of the current standard of care rather than an inert placebo, preserving ethical standards by not withholding proven therapies. When a placebo is used, it is typically added on top of SOC; for example, patients in both arms receive standard chemotherapy, with only the experimental arm receiving the novel agent and the control arm receiving placebo. This design maintains patient safety and equipoise while allowing for blinding, which reduces expectation bias among patients and investigators (Temple & Ellenberg, 2000).
The presence of a placebo or active control arm also informs regulatory decision-making. Agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require well-controlled trials—preferentially randomized and double-blind—before approving new oncology drugs. Control arms provide the benchmark against which primary endpoints such as progression-free survival, overall survival, and response rates are measured, providing the statistical rigor necessary for robust conclusions (U.S. Food and Drug Administration, 2018).
Ethical considerations dictate that placebo use must not expose patients to undue harm. Thus, placebo alone is rarely used in life-threatening conditions when effective treatments exist; instead, placebos supplement standard therapy. In early-phase trials where no SOC exists, single-arm designs without placebo may be acceptable, but confirmatory Phase III studies almost always include a control arm to validate efficacy and safety (Emanuel et al., 2000).
Ultimately, well-designed control and placebo arms are foundational to the scientific integrity of oncology trials, ensuring that new treatments genuinely improve patient outcomes and justify incorporation into clinical practice.
Regulatory Oversight and Ethics Committees
Oncology clinical trials operate under rigorous regulatory frameworks to ensure patient safety, scientific validity, and ethical integrity. Before a trial may begin, sponsors must submit detailed protocols to national regulatory agencies—such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA)—demonstrating a favorable risk–benefit profile and compliance with Good Clinical Practice (GCP) standards (International Council for Harmonisation, 2016). Parallel review by Institutional Review Boards (IRBs) or Independent Ethics Committees (IECs) assesses the study’s ethical merits, informed-consent processes, and safeguards for vulnerable populations (World Medical Association, 2013).
During the trial, regulatory authorities and IRBs/IECs monitor safety reports, protocol amendments, and site inspections to verify adherence to approved procedures. Sponsors must report serious adverse events to regulators within strict timelines and maintain complete, auditable records. Additionally, many trials utilize a Data Safety Monitoring Board (DSMB) to provide independent, ongoing safety oversight and recommend early trial termination if unexpected harms or overwhelming benefits arise (Ellenberg et al., 2003). Together, these layers of oversight uphold participant welfare and the credibility of oncology research.
Data Collection, Analysis, and Reporting
Accurate data collection, rigorous analysis, and transparent reporting are critical to the success and credibility of oncology clinical trials. During the trial, source data—from patient medical records, laboratory tests, imaging studies, and patient‐reported outcomes—are recorded on case report forms (CRFs) or directly into electronic data capture (EDC) systems. To ensure consistency, data are monitored through routine site visits and centralized reviews, verifying that procedures follow the protocol and Good Clinical Practice (GCP) standards (International Council for Harmonisation, 2016).
Once data are locked, statisticians perform analyses according to a pre–specified statistical analysis plan, using methods such as Kaplan‐Meier estimation for time‐to‐event endpoints (e.g., progression‐free and overall survival) and Cox proportional hazards models to estimate hazard ratios and confidence intervals (Therasse et al., 2000). Objective response rates are assessed per standardized criteria (e.g., RECIST), with blinded independent central review when required to minimize bias (Eisenhauer et al., 2009).
Transparent reporting follows guidelines such as CONSORT (Consolidated Standards of Reporting Trials), which mandate detailed descriptions of trial design, patient flow, baseline characteristics, efficacy and safety outcomes, and any protocol deviations (Schulz et al., 2010). Full disclosure of methods and results enables peer review, regulatory scrutiny, and meta‐analyses, ultimately translating trial findings into clinical practice and improving patient care.
Real-World Examples: Success Stories
Several pioneering institutions and companies have translated AI innovations into genuine improvements in oncology care:
Google Health and Breast Cancer Screening
In a landmark international study, Google Health’s deep learning model analyzed mammograms from over 25,000 women and outperformed human radiologists by reducing false negatives by up to 9.4% and false positives by 5.7% in the UK cohort, leading to earlier detection and fewer unnecessary recalls (McKinney et al., Nature, 2020).
Paige AI in Prostate Pathology
Paige AI’s prostate cancer detection platform received FDA breakthrough designation after demonstrating 97% sensitivity in identifying clinically significant prostate adenocarcinoma on digitized biopsy slides. Deployment across multiple pathology labs has accelerated diagnoses and standardized interpretations (Campanella et al., Nature Medicine, 2019).
Arterys in Lung Cancer Imaging
Arterys’ cloud-based AI tool for CT lung nodule detection received FDA clearance in 2018. In clinical use, it has reduced average radiologist reading time by 27% while maintaining diagnostic accuracy, enabling faster triage and earlier intervention for patients with suspected lung cancer (Scholten et al., Radiology, 2021).
Tempus and Precision Oncology
Tempus integrates genomic sequencing and clinical data with AI-driven analytics to guide treatment choices. In a real-world analysis, patients whose care aligned with Tempus recommendations experienced a 16% improvement in progression‐free survival, illustrating AI’s potential to personalize therapy (Ozenne et al., JCO Clinical Cancer Informatics, 2022).
These success stories demonstrate how AI tools—when rigorously validated and thoughtfully integrated—can enhance detection, diagnosis, and treatment selection, ultimately improving outcomes for cancer patients.
Patient Support and Resources
Navigating a cancer diagnosis and treatment journey can be overwhelming for patients and their families. AI-driven tools are increasingly integrated into patient support programs and digital resources to enhance education, self-management, and access to care.
Symptom Tracking and Management Apps
Mobile applications powered by AI allow patients to report symptoms in real time, receive personalized guidance, and flag concerning changes to their care teams. Studies have shown that routine symptom monitoring via apps can reduce emergency department visits and improve quality of life (Basch et al., 2016, Journal of Clinical Oncology). Examples include the PRO-CTCAE platform and FDA-cleared apps that interface with electronic health records to ensure timely clinical intervention.
Virtual Navigators and Chatbots
AI chatbots such as CancerChat123 and the Cancer Support Chatbot by the National Cancer Institute use natural language processing to answer common questions about side effects, appointment scheduling, and coping strategies. These virtual navigators provide 24/7 access to reliable information and direct patients to appropriate clinical or social support services (Tay et al., 2019, Psycho‐Oncology).
Telehealth and Remote Monitoring
During and after treatment, AI-enhanced telehealth platforms can triage calls, prioritize urgent issues, and analyze patient-reported data to predict complications before they escalate. A randomized trial demonstrated that AI-supported remote monitoring reduced hospitalizations by 30% in advanced cancer patients (Basch et al., 2020, JCO Oncology Practice).
Educational Portals and Decision Aids
Web-based decision aids, enriched by AI, adapt to a patient’s health literacy and emotional needs, helping them understand treatment options, potential outcomes, and side-effect management. Tools like the ASCO Patient Guide incorporate interactive videos and personalized risk calculators to support shared decision-making (Chien et al., 2021, Cancer).
The Future of Clinical Trials: Adaptive Designs & AI
Clinical trials are evolving beyond the traditional fixed‐design framework to embrace adaptive trial designs and advanced AI-driven analytics, enabling more efficient, ethical, and patient‐centric research.
Adaptive Trial Designs
Adaptive designs allow pre‐planned modifications to key trial parameters—such as sample size, randomization ratios, or treatment arms—based on interim data analyses. This flexibility can reduce patient exposure to ineffective therapies, accelerate identification of successful regimens, and lower overall trial costs. For example, the I-SPY 2 breast cancer trial uses a multi‐arm, adaptive platform to seamlessly add or drop therapeutic agents based on ongoing biomarker and outcome data, achieving rapid evaluation of multiple drugs in parallel (Barker et al., 2009, Journal of Clinical Oncology).
Seamless Phase Transitions
Another innovation is the “seamless” Phase II/III trial, where a single study transitions from proof‐of-concept to confirmatory testing without interruption, guided by interim efficacy signals. This approach can shorten development timelines and reduce administrative burdens associated with separate trials (Parkinson & Jaki, 2015, PLoS Medicine).
AI-Driven Patient Selection and Endpoints
AI algorithms are streamlining trial conduct by optimizing patient selection through predictive models that match individuals to experimental therapies based on molecular profiles and prior treatment history. AI also supports real‐time safety monitoring and endpoint adjudication, automatically detecting adverse events in electronic health records and imaging data (Rathore et al., 2019, The Lancet Digital Health).

Read More About AI in Oncology on OncoDaily
Digital and Decentralized Trials
The integration of mobile health (mHealth) devices, telemedicine, and e-consent platforms—augmented by AI‐powered data validation—enables decentralized trials, reducing patient travel and enhancing retention. Decentralized models broaden access, particularly in underserved communities, and generate real-world evidence alongside controlled trial data (Weldon et al., 2020, JCO Clinical Cancer Informatics).
Regulatory Acceptance and Collaboration
Regulatory bodies, including the FDA and EMA, are issuing guidance to support adaptive and AI-enabled trials, emphasizing robust statistical planning, transparency, and patient safety (FDA, 2019). Collaborative networks and data‐sharing consortia further accelerate innovation by providing standardized infrastructure and real-world datasets for AI model development.
By combining adaptive methodologies with AI’s predictive power, the next generation of clinical trials promises faster, smarter, and more patient-centered drug development—bringing breakthroughs to cancer patients more efficiently than ever before.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


