Chemotherapy precautions are essential but often overlooked. While patients receiving chemotherapy are generally aware of common side effects, less attention is paid to behavioral and environmental factors that can exacerbate toxicity or reduce treatment efficacy. This article addresses evidence-based contraindicated actions during chemotherapy, emphasizing their pathophysiological rationale, potential complications, and impact on pharmacokinetics or immune function.
Environmental Exposure and Infection Risk: Neutropenic Precautions During Chemotherapy
Chemotherapy-induced myelosuppression leads to a substantial reduction in circulating neutrophils, impairing the host’s ability to mount an effective innate immune response. This immunosuppressive state is particularly pronounced during the expected nadir period, typically occurring 7 to 14 days after chemotherapy administration, when patients are most vulnerable to opportunistic infections.
To mitigate the risk of infectious morbidity, patients should avoid crowded public spaces such as shopping centers, public transportation, and daycare facilities, where pathogen exposure is heightened. Environments with inadequate ventilation further compound this risk by facilitating airborne transmission of respiratory viruses. Additionally, contact with individuals exhibiting signs of infection, including household members, should be minimized.
Particular attention should be given to exposure to pets that may serve as reservoirs for zoonotic pathogens; handling of cat litter, reptile enclosures, or bird cages is strongly discouraged. In cases of profound neutropenia or febrile episodes, hospital-based protective isolation using positive pressure rooms and strict hygiene protocols may be warranted.
The importance of adhering to neutropenic precautions is underscored by infection prevention guidelines from the American Society of Clinical Oncology (ASCO) and the Centers for Disease Control and Prevention (CDC), which emphasize proactive environmental and behavioral modifications as essential components of supportive care during chemotherapy.

Unregulated Supplement Use: Pharmacokinetic Interference and Chemotherapy Efficacy
The use of dietary supplements, particularly herbal remedies and antioxidant formulations, poses significant and often underrecognized risks for patients undergoing chemotherapy. Many of these compounds exert pharmacologic activity capable of altering the metabolism, distribution, or efficacy of anticancer agents. Notably, several herbal products modulate cytochrome P450 (CYP) enzyme systems, with St. John’s Wort being a potent inducer of CYP3A4. This enzymatic induction can accelerate the metabolism of chemotherapeutic drugs such as irinotecan or docetaxel, leading to subtherapeutic plasma levels and reduced treatment efficacy. Additionally, supplements that modulate drug transporters, including P-glycoprotein, may alter intracellular drug concentrations and resistance profiles.
Antioxidants such as high-dose vitamin C and vitamin E are of particular concern, as they may counteract the oxidative stress mechanisms through which many chemotherapeutics—such as anthracyclines, platinum agents, and alkylating compounds—exert cytotoxic effects on tumor cells. Consequently, the indiscriminate use of such agents has the potential not only to diminish antitumor activity but also to confound toxicity profiles.
Given these risks, it is imperative that oncology providers routinely inquire about all over-the-counter products, herbal preparations, and nutritional supplements. Patients should be explicitly advised against initiating or continuing any supplement without prior consultation, as even seemingly benign agents may interact with treatment regimens in unpredictable and clinically meaningful ways.
Photosensitivity and UV Exposure: Cutaneous Toxicity in Chemotherapy Patients
Photosensitivity is a well-documented adverse effect associated with several chemotherapeutic agents, including fluorouracil, methotrexate, and dacarbazine. These drugs can enhance the skin’s susceptibility to ultraviolet (UV) radiation, leading to exaggerated inflammatory responses, erythema, blistering, and, in some cases, long-term pigmentary alterations or dermal injury.
The underlying mechanism involves drug-induced accumulation of photoreactive metabolites in the skin, which upon exposure to UVA or UVB radiation, generate reactive oxygen species and trigger direct cellular damage. This process can result in both acute phototoxic reactions and chronic photosensitive dermatitis, depending on cumulative sun exposure and individual skin sensitivity. Dermatologic recovery is often prolonged in this context, as chemotherapy itself impairs normal skin repair mechanisms. Accordingly, current dermatologic and oncologic guidelines emphasize the importance of strict photoprotection during chemotherapy.
Patients should avoid sun exposure during peak UV hours (typically 10 AM to 4 PM), apply broad-spectrum sunscreens with a minimum SPF of 50, and wear protective clothing, including wide-brimmed hats and UV-blocking garments. Importantly, these precautions should extend several weeks beyond the completion of treatment, particularly in regimens associated with prolonged photosensitization. Comprehensive patient education regarding sun safety is essential to prevent avoidable morbidity and preserve skin integrity during cytotoxic therapy.
Live Vaccinations and Immunization Timing: Navigating Safety in Immunosuppressed Hosts
The administration of live attenuated vaccines during chemotherapy is contraindicated due to the significant risk of uncontrolled viral replication in the setting of treatment-induced immunosuppression. Live vaccines such as measles-mumps-rubella (MMR), varicella, and yellow fever rely on a competent immune system to generate a protective response while containing viral replication. In chemotherapy patients with neutropenia, lymphocyte depletion, or compromised cellular immunity, this balance is disrupted, potentially leading to severe or disseminated infections.
Instead, inactivated vaccines—such as those targeting influenza, pneumococcus, and SARS-CoV-2—are considered safe and are routinely recommended for cancer patients. However, immunogenicity may be reduced during active chemotherapy, with lower seroconversion rates observed, particularly in patients receiving lymphocyte-depleting regimens. For optimal vaccine efficacy, guidelines from the Centers for Disease Control and Prevention (CDC), World Health Organization (WHO), and major oncology societies recommend administering routine immunizations at least two weeks before the initiation of cytotoxic therapy, when feasible. If vaccination is not possible prior to treatment, some vaccines may be deferred until hematologic recovery or between chemotherapy cycles, depending on risk-benefit assessment.
Additionally, household members and caregivers should be appropriately vaccinated to provide a cocooning effect and reduce transmission risk to the immunocompromised patient. Incorporating vaccine planning into oncology care pathways is essential to prevent avoidable infections and ensure comprehensive supportive management during chemotherapy.
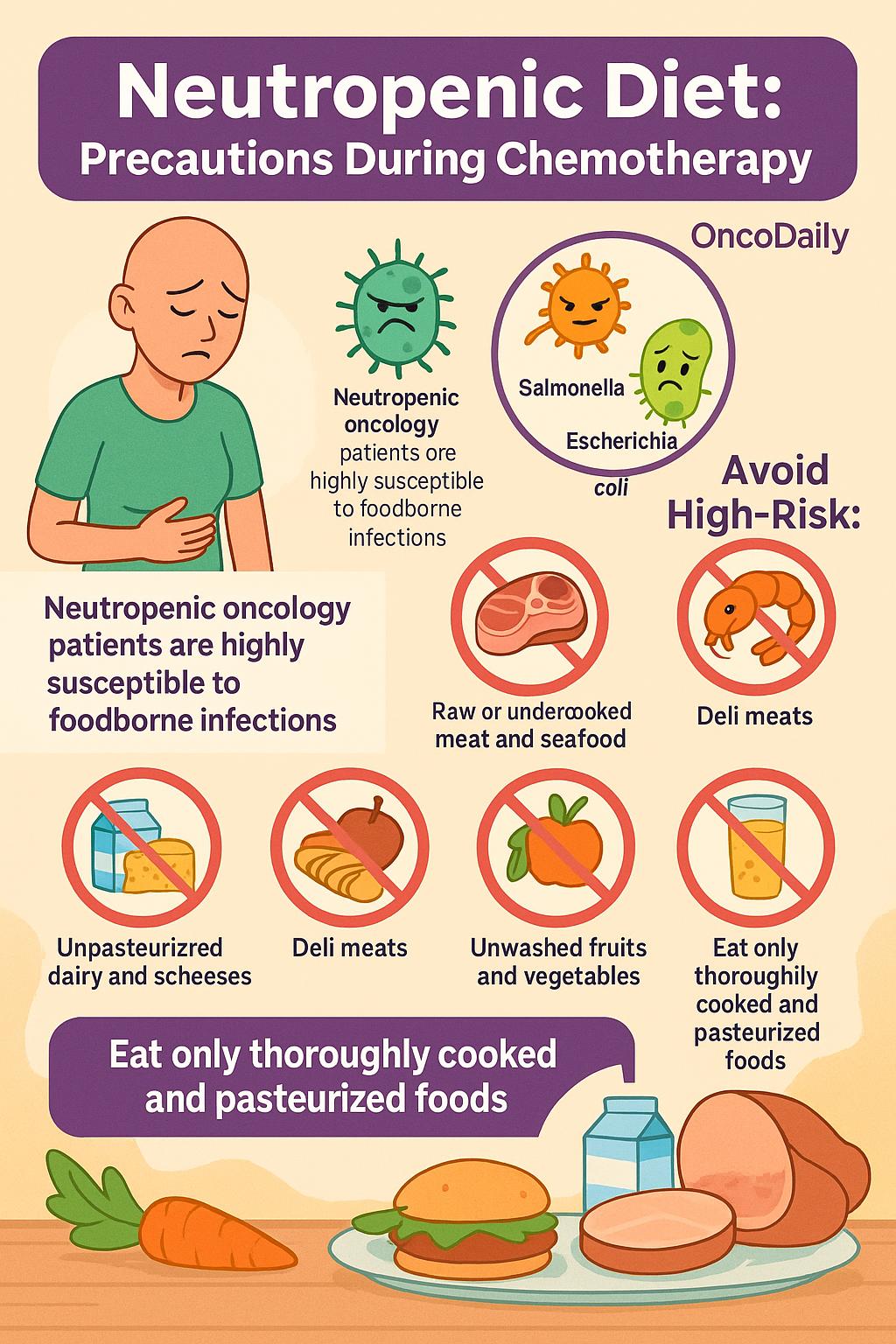
Foodborne Pathogens and Dietary Risk: Neutropenic Diet Recommendations During Chemotherapy
Neutropenia and gastrointestinal mucositis are frequent complications of chemotherapy that collectively compromise mucosal barrier integrity and impair immune surveillance, rendering patients highly susceptible to foodborne infections. In this vulnerable population, ingestion of contaminated or improperly prepared food can lead to severe and potentially life-threatening bacteremia or sepsis.
Common culprits include Listeria monocytogenes, Salmonella spp., and Escherichia coli, all of which have been implicated in outbreaks among immunocompromised hosts, including oncology patients. These pathogens are most frequently associated with high-risk foods such as unpasteurized dairy products, soft cheeses, raw or undercooked meat and seafood (e.g., sushi, oysters), deli meats, unwashed fruits and vegetables, and unpasteurized juices.
The adoption of a neutropenic diet—characterized by strict food hygiene, avoidance of raw animal products, and consumption of only thoroughly cooked and pasteurized items—is a widely endorsed precautionary strategy during periods of profound immunosuppression. While the evidence for routine use of neutropenic diets has evolved, major oncology centers and infectious disease guidelines continue to support targeted dietary restrictions during high-risk treatment phases.
Comprehensive patient education on food handling, storage, and preparation is essential, and collaboration with dietitians can ensure nutritional adequacy while minimizing infectious risk. These practices are integral to supportive care in oncology, particularly for patients undergoing intensive cytotoxic regimens or hematopoietic stem cell transplantation.
Exercise Caution: Balancing Physical Activity and Safety During Chemotherapy
While physical activity is widely recognized for its benefits in reducing cancer-related fatigue, preserving functional capacity, and supporting mental well-being, the intensity and type of exercise must be carefully tailored during chemotherapy. Moderate, low-impact activities such as walking, gentle yoga, and stretching can enhance circulation, mitigate deconditioning, and improve mood without imposing undue physiological stress.
However, vigorous or high-impact exercise during active chemotherapy may exacerbate anemia, intensify fatigue, and hinder recovery from cytopenias. In particular, patients with metastatic involvement of the skeletal system face an elevated risk of pathologic fractures, especially in weight-bearing bones or sites with lytic lesions. Activities involving heavy lifting, high-velocity movements, or contact sports should be avoided in this context. Moreover, chemotherapy-induced peripheral neuropathy may impair coordination and proprioception, further increasing fall risk during unsupervised physical exertion. As such, individualized exercise prescriptions should be developed in collaboration with oncology-trained physiotherapists or rehabilitation specialists.
These professionals can assess baseline functional status, treatment-related limitations, and specific cancer-related risks to design safe, adaptive regimens. Emerging guidelines from the American College of Sports Medicine (ACSM) and oncology rehabilitation literature emphasize a personalized approach to exercise during cancer treatment, ensuring that the benefits of physical activity are maximized without compromising patient safety.
Adherence to Chemotherapy Regimens: Risks of Dose Omission and Self-Modification
The efficacy of chemotherapy is critically dependent on adherence to carefully structured dosing schedules that are derived from pharmacokinetic modeling, tumor growth kinetics, and established clinical trial data. These regimens are calibrated to maximize tumor cytotoxicity while allowing sufficient recovery time for healthy tissue.
Deviations from prescribed schedules—whether through skipped doses, unsanctioned treatment delays, or patient-led dose modifications—can compromise therapeutic efficacy and contribute to suboptimal outcomes. Non-adherence has been associated with reduced tumor response rates, accelerated development of drug resistance, and increased risk of disease progression. Additionally, attempting to “catch up” on missed doses or reschedule infusions without proper clinical oversight may result in cumulative toxicity or unanticipated adverse events. This is particularly relevant in oral chemotherapy regimens, where patient-controlled administration has introduced new challenges in monitoring adherence.
Studies have shown that adherence rates to outpatient oral regimens may fall below 80%, with documented impacts on progression-free and overall survival in cancers such as breast, colorectal, and chronic myeloid leukemia. Oncology providers must emphasize the importance of strict adherence to treatment protocols and proactively address barriers such as side effect management, logistical constraints, and psychosocial factors.
Alcohol, Cannabis, and Recreational Drugs: Hepatic and Neurocognitive Risks During Chemotherapy
The concurrent use of alcohol, cannabis, or recreational drugs during chemotherapy introduces significant risks that may potentiate toxicity, interfere with drug metabolism, and obscure adverse event detection. Alcohol consumption, even in moderate quantities, can exacerbate hepatotoxicity in patients receiving chemotherapeutic agents known for hepatic metabolism or hepatic injury, such as methotrexate, capecitabine, and anthracyclines.
These agents rely on functional hepatic clearance pathways, including CYP450 enzymes and conjugation reactions, which are impaired by alcohol-induced liver inflammation and enzyme induction. Chronic alcohol use may also downregulate hepatic glutathione reserves, further increasing vulnerability to oxidative damage. Similarly, cannabinoids—including both medical and recreational cannabis—can interact with hepatic enzyme systems, particularly CYP3A4 and CYP2C9, altering the pharmacokinetics of chemotherapeutic drugs and immunotherapies.
Moreover, the sedative and antiemetic effects of cannabis and illicit substances may mask early symptoms of toxicity, such as nausea, dizziness, or confusion, delaying appropriate medical intervention. Neurocognitive impairment associated with recreational drug use can also interfere with medication adherence, informed decision-making, and accurate symptom reporting.
While certain cannabinoids have demonstrated palliative benefits in cancer-related pain, appetite loss, and chemotherapy-induced nausea, such interventions must be supervised and appropriately dosed within evidence-based clinical frameworks. The unsupervised use of cannabis extracts or street-acquired substances lacks consistency in formulation, potency, and safety, posing serious risks during cytotoxic treatment.
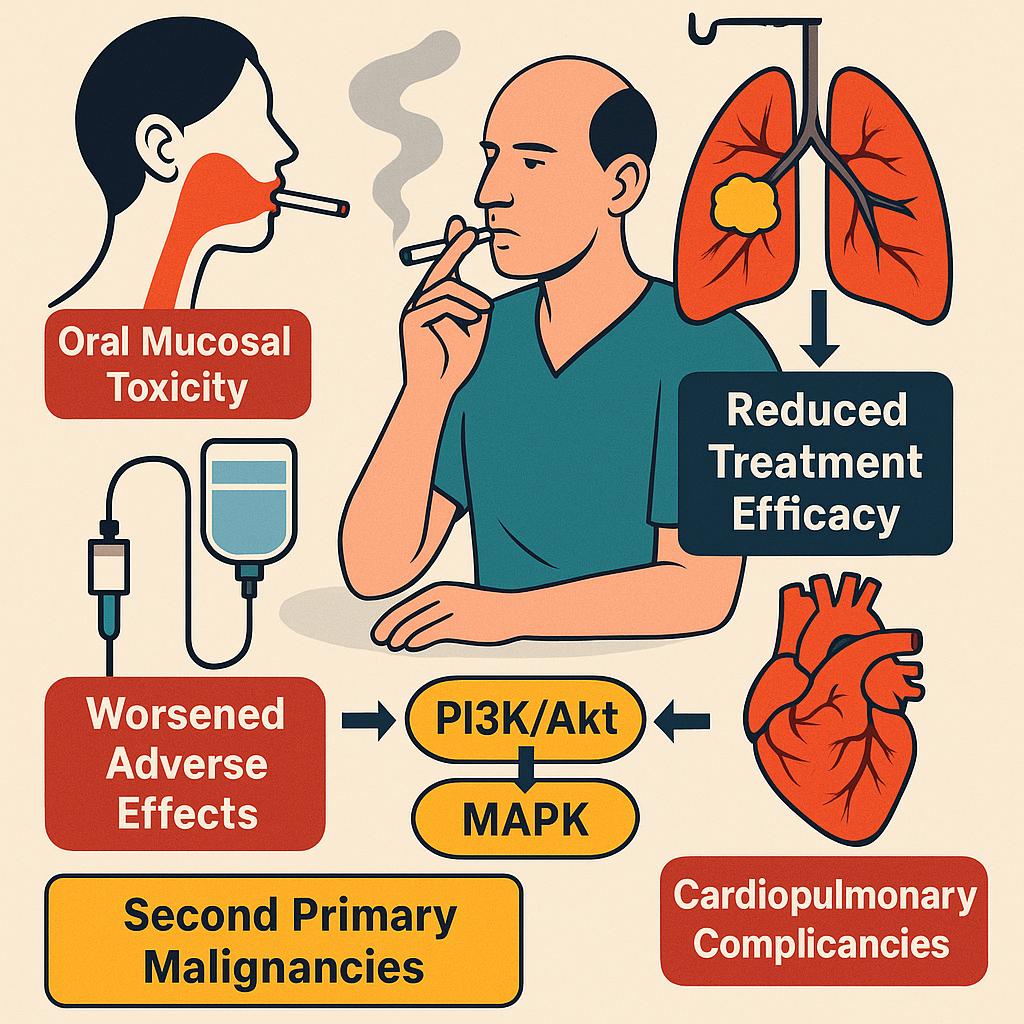
Tobacco Use During Chemotherapy: Impact on Efficacy, Toxicity, and Long-Term Outcomes
Ongoing tobacco use during chemotherapy is a modifiable risk factor that significantly worsens treatment-related toxicity and compromises oncologic outcomes. Nicotine and tobacco-related carcinogens promote oxidative stress, chronic inflammation, and endothelial dysfunction, which collectively exacerbate chemotherapy-induced adverse effects such as mucositis and vascular injury. In particular, oral mucosal toxicity is frequently intensified among smokers receiving mucotoxic regimens.
Beyond toxicity, tobacco use directly interferes with treatment efficacy. Multiple studies have demonstrated that continued smoking during therapy is associated with reduced response rates and poorer survival outcomes, especially in malignancies such as lung, head and neck, and bladder cancer. Mechanistically, tobacco smoke induces hepatic cytochrome P450 enzymes, notably CYP1A1 and CYP1A2, which can accelerate the metabolism of chemotherapeutic agents and reduce their systemic exposure. In parallel, nicotine-mediated activation of prosurvival signaling pathways—such as PI3K/Akt and MAPK—may promote tumor resistance to both cytotoxic and targeted agents.
Furthermore, persistent smoking increases the risk of cardiopulmonary complications, impairs wound healing, and contributes to the development of second primary malignancies. These effects are particularly detrimental in patients receiving radiation therapy or undergoing surgery as part of multimodal treatment.
Given these risks, clinical guidelines from the National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology (ASCO), and the U.S. Surgeon General recommend integrating tobacco cessation into routine oncology care. Effective cessation strategies include behavioral counseling, nicotine replacement therapy, and pharmacologic agents such as bupropion and varenicline, all of which are safe for use during chemotherapy.
Travel During Chemotherapy: Clinical Risks and Precautionary Planning
Traveling during chemotherapy requires careful consideration due to the heightened vulnerability of oncology patients to medical complications and limited access to urgent care services in unfamiliar environments. Cytotoxic agents often induce immunosuppression, anemia, and gastrointestinal side effects that can be exacerbated by the physiological stressors associated with long-distance travel, particularly air travel.
One major concern is the increased risk of venous thromboembolism (VTE), a well-documented complication in patients with malignancy—especially those receiving chemotherapy, who face an elevated baseline risk. Prolonged immobility during travel, dehydration, and underlying prothrombotic states further compound this risk. Thromboprophylaxis may be indicated for high-risk patients, particularly those with prior VTE, advanced disease, or central venous catheters. Low-molecular-weight heparin or direct oral anticoagulants may be considered in select cases, based on individualized risk-benefit assessments.
Air travel may also intensify symptoms of chemotherapy-induced anemia, leading to increased fatigue, dizziness, or dyspnea due to lower oxygen cabin pressure. Additionally, the confined environment of airports and airplanes increases exposure to respiratory and gastrointestinal pathogens, which poses a particular threat to immunocompromised individuals. Patients with recent neutropenia or mucositis are especially susceptible to infection and may require prophylactic measures or travel postponement.
Pre-travel medical evaluation is essential to assess treatment schedules, hematologic status, recent toxicities, and the stability of comorbid conditions. Oncologists should advise patients on medication transport, emergency contact information, and the location of nearby oncology centers at their destination. Travel insurance policies with provisions for cancer-related complications, treatment delays, and medical evacuation should be secured prior to departure. When deemed appropriate, travel can be safely undertaken with comprehensive planning, physician guidance, and access to supportive resources.
Sexual Activity and Reproductive Safety: Managing Risks During Chemotherapy
Chemotherapy can have profound implications for both sexual health and reproductive safety, necessitating clear guidance and proactive counseling for patients undergoing treatment. Several chemotherapeutic agents, including alkylating agents and platinum-based compounds, are excreted in bodily fluids such as semen, vaginal secretions, saliva, and urine for up to 48–72 hours following infusion. Unprotected sexual activity during this window may expose partners to cytotoxic metabolites, posing theoretical risks of local irritation, mutagenic effects, or systemic absorption, particularly through mucosal surfaces.
In addition to transmission concerns, many chemotherapeutic agents are gonadotoxic and teratogenic, with the potential to damage germ cells and disrupt reproductive hormones. These effects may lead to temporary or permanent infertility, impaired spermatogenesis or oogenesis, and increased risk of congenital anomalies in the event of conception during treatment. As such, effective contraception is essential throughout the entire course of chemotherapy—and for a period afterward—even in patients who are amenorrheic or presumed infertile.
Current guidelines recommend the consistent use of barrier protection (e.g., condoms) during all forms of sexual activity, not only to protect partners from exposure to chemotherapeutic residues but also to reduce infection risk in immunosuppressed patients. Condom use should be maintained for at least 7 days after oral chemotherapy and up to 48–72 hours following intravenous regimens, depending on the agent’s elimination profile.
Fertility preservation should be addressed prior to treatment initiation, especially in young adults and patients receiving regimens with high gonadotoxic potential. Options include sperm banking, oocyte or embryo cryopreservation, and, in certain cases, ovarian tissue preservation. Referral to a reproductive endocrinologist should be offered promptly when time allows.
Sexual health counseling should also address intimacy concerns, libido changes, and physical discomfort, all of which may be affected by treatment side effects such as fatigue, mucositis, or hormonal shifts. By providing tailored education on sexual safety and reproductive health, oncology teams can support both the physical and psychosocial well-being of patients during chemotherapy.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What should I avoid during chemotherapy?
Avoid crowded places, unwashed produce, alcohol, tobacco, and unapproved supplements.
Can I take supplements or vitamins while on chemo?
No, unless approved by your oncologist—they can interfere with drug metabolism.
Is it safe to go out in the sun during chemotherapy?
Many chemo drugs cause photosensitivity—always use sun protection.
Can I get vaccinated during chemotherapy?
Avoid live vaccines; ask your doctor about safe options and timing.
Why should I avoid certain foods during chemo?
To prevent foodborne infections due to weakened immune defenses.
Is it okay to exercise during chemo?
Yes, but only light or moderate activity recommended by your doctor.
Can I travel while on chemotherapy?
Only with prior medical approval and planning for emergencies.
How does chemo affect fertility?
It may cause temporary or permanent infertility—ask about preservation options.
Can I have sex during chemo?
Yes, with barrier protection and precautions for body fluid exposure.
Why should I stop smoking during chemotherapy?
Smoking reduces treatment effectiveness and increases side effects.