Advanced non-small cell lung cancer (NSCLC) continues to pose a significant clinical challenge, particularly after disease progression on first-line treatment. With the emergence of cancer vaccines as a novel immunotherapeutic approach, a systematic review and meta-analysis was conducted to evaluate their efficacy and safety in patients with advanced NSCLC following first-line therapy. This study, which included 11 randomized controlled trials involving a total of 3228 patients, aimed to provide high-level evidence for the use of cancer vaccines in this setting, with a focus on survival outcomes, treatment-related adverse events, and the role of immune response as a predictive biomarker.
Published in eClinicalMedicine, Aug 2025
Methods and Study Design
This study was conducted as a systematic review and meta-analysis registered with the PROSPERO database (registration number CRD42024568178). The authors performed a comprehensive literature search across PubMed, the Cochrane Library, and Embase databases, covering publications up to December 27, 2024. The search aimed to identify phase II or III randomized controlled trials (RCTs) that evaluated the use of cancer vaccines in patients with advanced or metastatic non-small cell lung cancer (NSCLC) who had received prior first-line therapy.
Eligible studies were required to meet several inclusion criteria: they had to be full-text RCTs, involve adult patients with stage III or IV NSCLC, administer cancer vaccines after first-line treatment, and report on survival outcomes. Trials were excluded if they were phase I studies, lacked endpoint data, were published in languages other than English, or focused on other therapeutic comparators rather than vaccines.
The primary endpoint of this analysis was overall survival (OS), while secondary outcomes included progression-free survival (PFS) and treatment-related adverse events (TRAEs). To synthesize data, the authors used a random-effects model, accounting for variability across studies. Subgroup analyses were conducted to explore survival benefits in specific patient populations, and exploratory analyses assessed the potential of vaccine-induced immune responses as predictive biomarkers. The methodological quality and risk of bias for included trials were assessed using the Cochrane Risk of Bias tool (RoB 2), while the overall quality of the meta-analysis was evaluated using the AMSTAR 2 checklist. The certainty of evidence for each outcome was further graded using the GRADE system.
Results
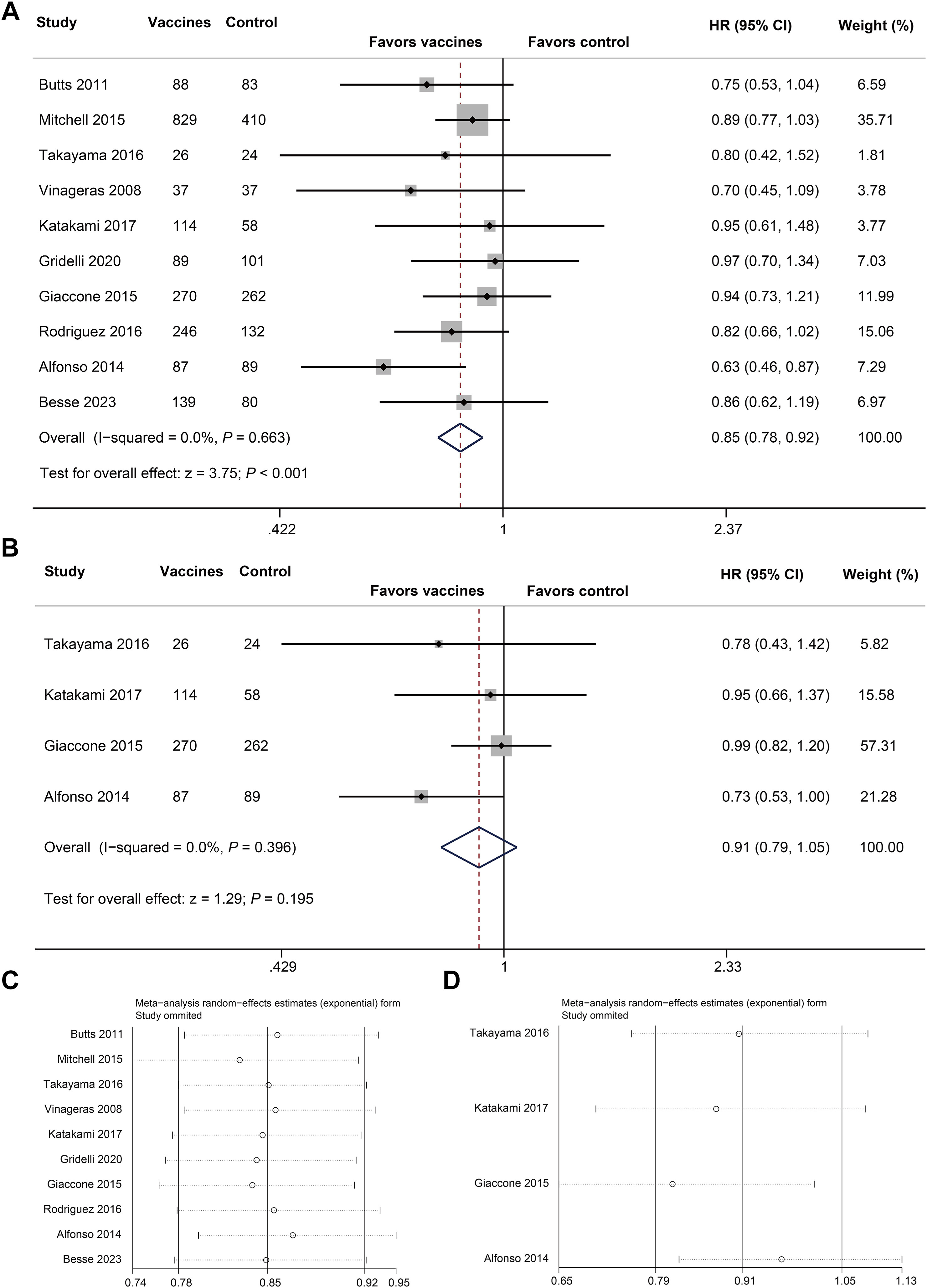
Overall Survival (OS): Cancer vaccines significantly improved OS compared to control treatments.
- HR = 0.85, 95% CI: 0.78–0.92, P < 0.001
Progression-Free Survival (PFS): No statistically significant improvement in PFS.
- HR = 0.91, 95% CI: 0.79–1.05, P = 0.195
Subgroup OS Benefits:
- ECOG = 1: HR = 0.80 (95% CI: 0.68–0.95)
- Stage IV NSCLC: HR = 0.83 (95% CI: 0.70–0.98)
- First-line chemotherapy recipients: HR = 0.78 (95% CI: 0.66–0.93)
- Patients with squamous cell carcinoma: HR = 0.74 (95% CI: 0.61–0.90)
- Stable disease after first-line therapy: HR = 0.78 (95% CI: 0.67–0.92)
- Current smokers: HR = 0.61 (95% CI: 0.46–0.81)
Safety: Cancer vaccines were well tolerated.
- No significant increase in all-grade or serious adverse events
- TRAEs: OR = 1.50 (95% CI: 0.63–3.61), P = 0.361
- Higher incidence of injection site reactions (OR = 14.97) and headache (OR = 1.75), both manageable
Biomarker Findings:
- Immune responders had significantly better OS than non-responders
- HR = 0.34 (95% CI: 0.24–0.50), P < 0.001
Key Findings
This meta-analysis revealed that cancer vaccines provide a significant overall survival benefit for patients with advanced non-small cell lung cancer (NSCLC) following first-line therapy. However, the analysis did not show a statistically significant improvement in progression-free survival, suggesting that the primary advantage of vaccine therapy lies in its impact on long-term survival rather than short-term disease control.
Importantly, the safety profile of cancer vaccines was favorable. No major increases in adverse events or serious adverse events were observed compared to control groups, supporting the tolerability of these therapies in the advanced disease setting.
Further subgroup analyses identified patient populations that were more likely to benefit from vaccine therapy. These included individuals with squamous cell carcinoma, those with an Eastern Cooperative Oncology Group (ECOG) performance status of 1, patients with a history of smoking, and those who achieved stable disease after first-line treatment. These findings highlight the potential to tailor vaccine strategies based on patient and tumor characteristics.
Additionally, vaccine-induced immune responses were associated with improved survival outcomes, suggesting that immunologic reactivity may serve as a useful predictive biomarker for identifying patients who are most likely to respond favorably to cancer vaccines.
Key Takeaway Messages
-
Cancer vaccines significantly prolong overall survival in patients with advanced NSCLC after first-line therapy.
-
The survival benefit is most pronounced in patients with squamous cell carcinoma, smoking history, ECOG 1, and those who had stable disease after initial treatment.
-
Progression-free survival was not significantly improved, highlighting the potential role of vaccines in modulating long-term outcomes rather than short-term tumor control.
-
The safety profile is favorable, with no major increase in treatment-related or serious adverse events.
-
Vaccine-induced immune responses strongly correlate with improved survival, supporting their use as a predictive biomarker for treatment efficacy.
-
These findings support the integration of cancer vaccines as a post-first-line or maintenance therapy in selected advanced NSCLC populations and provide a foundation for personalized immunotherapy approaches.
Conclusion
This systematic review and meta-analysis of 11 randomized trials provides strong evidence that cancer vaccines can significantly prolong survival and are well-tolerated in patients with advanced NSCLC after first-line therapy. The benefit appears greater in patients with squamous cell histology, prior smoking history, and stable disease, and those with a detectable immune response to the vaccine. While not improving PFS, cancer vaccines offer a compelling survival and safety advantage, supporting their clinical application as a post-first-line or maintenance strategy. Future studies should focus on refining biomarker-driven patient selection and integrating vaccines with modern immunotherapy regimens.
You can read the full abstract here
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD, Editor-in-Chief of OncoDaily IO
