What is Chordoma?
Chordoma is a rare type of cancer that occurs in the bones of the skull base and spine. It arises from remnants of the notochord, a structure present during embryonic development. Although chordomas are slow-growing, they are locally aggressive and can cause significant morbidity due to their location near critical structures. Pediatric chordomas, which account for a small percentage of all chordoma cases, present unique challenges in diagnosis and treatment.
Epidemiology
Chordoma is an exceptionally rare tumor, with an annual incidence of approximately 1 per 1,000,000 people in the United States and Europe, leading to about 350 cases per year in the U.S. Pediatric chordomas are even rarer, accounting for less than 5% of all chordoma cases. Most pediatric chordomas occur in the cranium, particularly at the skull base, and are more common in males than females.
Causes and Risk Factors
The exact cause of chordoma is not well understood, but several genetic factors have been implicated. Pediatric chordomas are more likely to be driven by germline mutations, which can be inherited and increase the risk of cancer. Key genetic findings include:
- ARID1B Gene Mutations: Found in a significant number of pediatric chordoma cases, these mutations are often of germline origin and are associated with the SWI/SNF chromatin remodeling complex (which regulates which genes are turned on or off).
- Mitochondrial DNA Mutations: Alterations in mitochondrial DNA, particularly in NADH (Mitochondrial Complex I genes), have been identified in pediatric chordoma samples.
In rare cases, this tumor can occur in multiple members of the same family, indicating a strong genetic predisposition. Some families with familial chordoma have been found to have an extra copy of the TBXT* gene, although this is not a common finding. There are no known environmental or lifestyle factors that increase the risk of developing chordoma. The vast majority of chordomas occur sporadically and are not linked to any specific external factors.
*TBXT gene guides the development of the spine and central nervous system during the early stages of growth.
Types
Chordomas are classified into three main types based on their histological characteristics:
- Conventional Chordoma: The most common type, characterized by cells with a bubbly appearance and expression of the brachyury protein.
- Poorly Differentiated Chordoma: A more aggressive form with a worse prognosis, often associated with loss of the SMARCB1* gene.
*SMARCB1 – gene is coding a protein involved in various intracellular processes, such as epigenetic regulation, cell cycle progression, cell proliferation, and differentiation.
Symptoms of Pediatric Chordoma
The symptoms of pediatric chordoma depend largely on the location of the tumor along the spine and its size. The most common presenting symptoms are:
Cranial (Skull Base) Chordomas
- Diplopia (double vision): This is one of the most frequent symptoms. It results from impingement on the cranial nerves controlling eye movements.
- Headaches: Headaches occur in around 40% of pediatric cranial chordoma cases, often due to increased intracranial pressure from the tumor mass.
- Cranial nerve palsies: Impairment of cranial nerves is seen in approximately 60% of cases. The sixth cranial nerve (abducens nerve) controlling lateral eye movement is most commonly affected.
- Long tract signs: Such as weakness, numbness or abnormal reflexes due to compression of the brainstem or spinal cord.
- Swallowing difficulties (dysphagia): Caused by involvement of the lower cranial nerves controlling swallowing muscles.
- Speech and voice abnormalities: Also resulting from lower cranial nerve deficits.
Spinal Chordomas
- Back pain: A common symptom, often the first sign of a spinal chordoma.
- Radicular pain: Shooting pain along the distribution of a spinal nerve root compressed by the tumor.
- Numbness, weakness or abnormal reflexes in the arms or legs: Due to compression of the spinal cord or nerve roots.
- Bowel and bladder dysfunction: Such as incontinence, retention, or constipation from involvement of autonomic nerves.
In young children under 5 years, increased intracranial pressure, long tract signs, and lower cranial nerve deficits are more common due to frequent inferior extension of cranial tumors.
Diagnosis
Diagnosing pediatric chordoma involves a combination of clinical evaluation, imaging studies, and biopsy. Given the tumor’s rarity and its potential to mimic other conditions, a thorough and systematic approach is essential.
Clinical Evaluation
The diagnostic process begins with a detailed medical history and physical examination. The physician will assess the patient’s symptoms, such as headaches, vision changes, back pain, or neurological deficits, and perform a physical exam to check for signs of disease, such as lumps or neurological abnormalities.
Imaging Studies
Imaging is crucial for diagnosing chordoma, determining its extent, and planning treatment. The following imaging modalities are commonly used:
- Magnetic Resonance Imaging (MRI): MRI is the preferred imaging technique for chordomas due to its superior soft tissue contrast. It helps delineate the tumor’s extent and its relationship with adjacent structures.
- Computed Tomography (CT) Scan: CT scans are useful for evaluating bone involvement and detecting calcifications within the tumor. Chordomas often appear as destructive, lytic lesions with possible sclerosis at the margins. CT can also help differentiate chordomas from other bone tumors.
- Plain Radiographs: While not as detailed as MRI or CT, plain radiographs can show the extent of bone involvement and the presence of a bulky soft tissue mass. They may reveal an ill-defined endosteal margin or lytic lesions.
- Bone Scans: Bone scans are sometimes used to assess the extent of the disease, although chordomas typically show normal to decreased uptake.
Biopsy
A biopsy is essential for confirming the diagnosis of chordoma. The biopsy procedure must be carefully planned to avoid seeding tumor cells along the biopsy tract, which should be included in the subsequent surgical resection to minimize the risk of local recurrence.
- Fine-Needle Aspiration (FNA): FNA is often preferred for preoperative diagnosis as it is less invasive and associated with lower local recurrence rates compared to open biopsy. The presence of physaliphorous cells with round nuclei, bland chromatin, and distinct cytoplasmic borders in a myxoid background is diagnostic of chordoma.
- Core Needle Biopsy or Open Biopsy: These methods may be used when FNA is inconclusive. The biopsy sample is examined histologically to confirm the presence of chordoma cells, which typically show notochordal differentiation and express the brachyury protein.
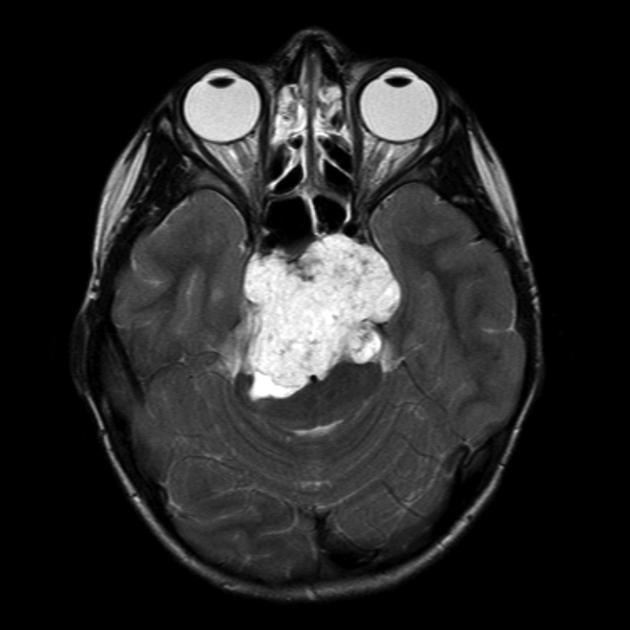
Chordoma of the clivus (bony ridge at the base of the skull) in an 8-year-old girl. The image is taken from radiopaedia.org.
Treatment
- Surgical Resection: Surgical resection is the primary treatment for chordoma. The goal is to remove as much of the tumor as possible while preserving neurological function. Complete surgical resection is often challenging due to the tumor’s proximity to critical structures. Gross-total resection (a surgical procedure aimed at removing the entire visible tumor) is associated with better outcomes.
- Radiation Therapy: Radiation therapy is commonly used as an adjuvant treatment following surgery. Proton beam therapy is preferred for pediatric patients due to its precision and reduced damage to surrounding tissues. High-dose conformal radiation therapy, such as proton beam radiation, is often employed to target residual tumor tissue.
- Chemotherapy: The role of chemotherapy in treating chordoma is limited and not well-established. It is generally reserved for cases with dedifferentiated or poorly differentiated chordomas, which are more aggressive and less responsive to conventional treatments.
- Targeted Therapy and Immunotherapy: Experimental studies are exploring the use of targeted therapies and immunotherapies for chordoma. These treatments target specific genetic mutations and molecular pathways involved in tumor growth. Clinical trials are ongoing to evaluate the efficacy of these novel therapies in pediatric chordoma patients.
More information about completed and ongoing clinical trials can be found here – clinicaltrials.gov.
Patient Survivorship
Survivorship care for pediatric chordoma patients is essential to address the long-term physical, emotional, and social challenges that arise after treatment. This care involves regular follow-up exams, health monitoring, and support services to ensure the best possible quality of life. Regular follow-up exams are crucial for monitoring recurrence and managing the late effects of treatment. These exams typically involve periodic MRI or CT scans to detect any signs of the tumor returning or spreading. Health monitoring also includes checking for issues that may arise months or years after treatment, such as late effects of radiation, heart disease, diabetes, or secondary cancers.
Problems During and After Treatment and How to Manage Them
- Pain Management: Pain is a common issue during and after chordoma treatment. Effective pain management strategies include medications such as NSAIDs, opioids, and adjuvant medications like antidepressants and anticonvulsants. Physical therapy can help improve mobility and reduce pain through exercises and manual therapy. Palliative care focuses on relieving symptoms and improving quality of life through a multidisciplinary approach.
- Fatigue: Cancer-related fatigue can be managed through regular exercise, ensuring good sleep practices, and proper nutrition. Tailored exercise programs can help improve energy levels and reduce fatigue, while good sleep hygiene and nutritional support can help maintain energy levels.
- Mobility Issues: Mobility issues can result from surgery or radiation therapy, particularly in cases involving the spine. Management strategies include physical therapy to improve strength, balance, and coordination, assistive devices such as braces, walkers, or wheelchairs to aid mobility, and occupational therapy to help patients adapt to daily activities and improve their independence.
- Sensory Deficits: Chordomas, especially those located at the skull base, can affect vision, hearing, and swallowing. Management includes vision and hearing rehabilitation through specialized therapies and assistive devices, and speech and swallowing therapy to address difficulties in speaking and swallowing, often involving exercises and techniques to improve function.
- Emotional and Cognitive Support: Survivors may experience significant emotional and cognitive challenges, including anxiety, depression, and cognitive impairments. Management strategies include psychological support through counseling and therapy, cognitive rehabilitation programs designed to improve cognitive function, and support groups to provide emotional support and practical advice.
- Social and Practical Support: Survivors often encounter social and practical challenges, like returning to school and coping with social isolation. Support strategies include accessing social work services to navigate practical challenges and joining support groups that offer a community of peers familiar with the unique challenges of chordoma survivorship.
In this video, Michael shares his experience as a chordoma patient. The video is produced by Texas Children’s Hospital.
Prognosis
The prognosis of pediatric chordoma varies based on several factors, including age at diagnosis, tumor location, histological subtype, and the extent of surgical resection.
The overall survival rates (indicate the percentage of patients who are still alive after a certain period following diagnosis or treatment) for children and adolescents with cranial chordomas range from 50% to 80%. Younger children, particularly those under 5 years of age, tend to have a worse prognosis compared to older children and adolescents. This is partly due to the more aggressive nature of the disease in younger patients.
Factors Influencing Prognosis
- Extent of Surgical Resection: A gross total resection is associated with significantly better outcomes. Near-total resection or subtotal resection is associated with higher recurrence rates and poorer outcomes.
- Histological Subtype: Histopathology is a crucial prognostic factor. Patients with classical chordoma generally have better outcomes compared to those with poorly differentiated chordomas. Chondroid chordomas have a variable prognosis, with some studies suggesting a more benign course compared to classical chordomas.
- Tumor Location: Tumors located at the skull base generally have a better prognosis than those located in the spine. This is due to the feasibility of achieving more complete resections and the effectiveness of adjuvant therapies like proton beam therapy in skull base tumors.
- Genetic Mutations: Genetic factors also play a significant role in the prognosis of pediatric chordoma. Mutations in genes such as ARID1B and mitochondrial DNA (mtDNA) aberrations have been identified as important in the genesis of chordoma. These genetic alterations can influence the behavior of the tumor and its response to treatment.
- Recurrence and Metastasis: Disease recurrence or progression and the presence of metastatic disease are associated with worse overall survival. Recurrence is common due to the tumor’s infiltrative nature and the difficulty in achieving complete resection.
Conclusion
Pediatric chordoma presents unique challenges, but advancements in diagnosis, treatment, and survivorship care offer hope and improved outcomes for young patients and their families. While the journey through diagnosis and treatment can be challenging, the comprehensive care provided by multidisciplinary teams ensures that each aspect of the patient’s well-being is addressed. Survivorship care is a critical component, focusing on regular monitoring, managing long-term side effects, and providing emotional and social support. With ongoing research and the development of new therapies, the future holds promise for even better management of this rare disease.
Families and caregivers play a vital role in supporting their loved ones through this journey, and access to resources and support groups can make a significant difference. By staying informed and proactive, patients and their families can navigate the challenges of this tumor with resilience and hope, looking forward to a future where they can lead fulfilling and healthy lives.
Sources
- American Cancer Society – cancer.org
- National Cancer Institute – cancer.gov
- American Brain Foundation
- American Society of Clinical Oncology – asco.org
- Children’s Hospital of Philadelphia – chop.edu
- Chordoma Foundation
- Paediatric Chordomas – Orphanet Journal of Rare Diseases
- Radiopaedia – radiopedia.org


