Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related mortality, largely due to late diagnosis, high recurrence, and resistance to therapy. A central hallmark of HCC biology is apoptosis resistance, which allows malignant hepatocytes to survive despite cytotoxic stress from systemic therapies. Increasing evidence implicates epigenetic dysregulation as a driver of this resistance, positioning epigenetic therapies (epi-drugs) as promising adjuncts to current treatment paradigms.
This review focuses on the c-FLIP/Ku70 cytoplasmic complex, an anti-apoptotic protein–protein interaction that stabilizes c-FLIP and blocks death receptor–mediated apoptosis. Disrupting this complex—particularly through epigenetic modulation—emerges as a compelling therapeutic strategy in HCC.
Mechanistic Rationale
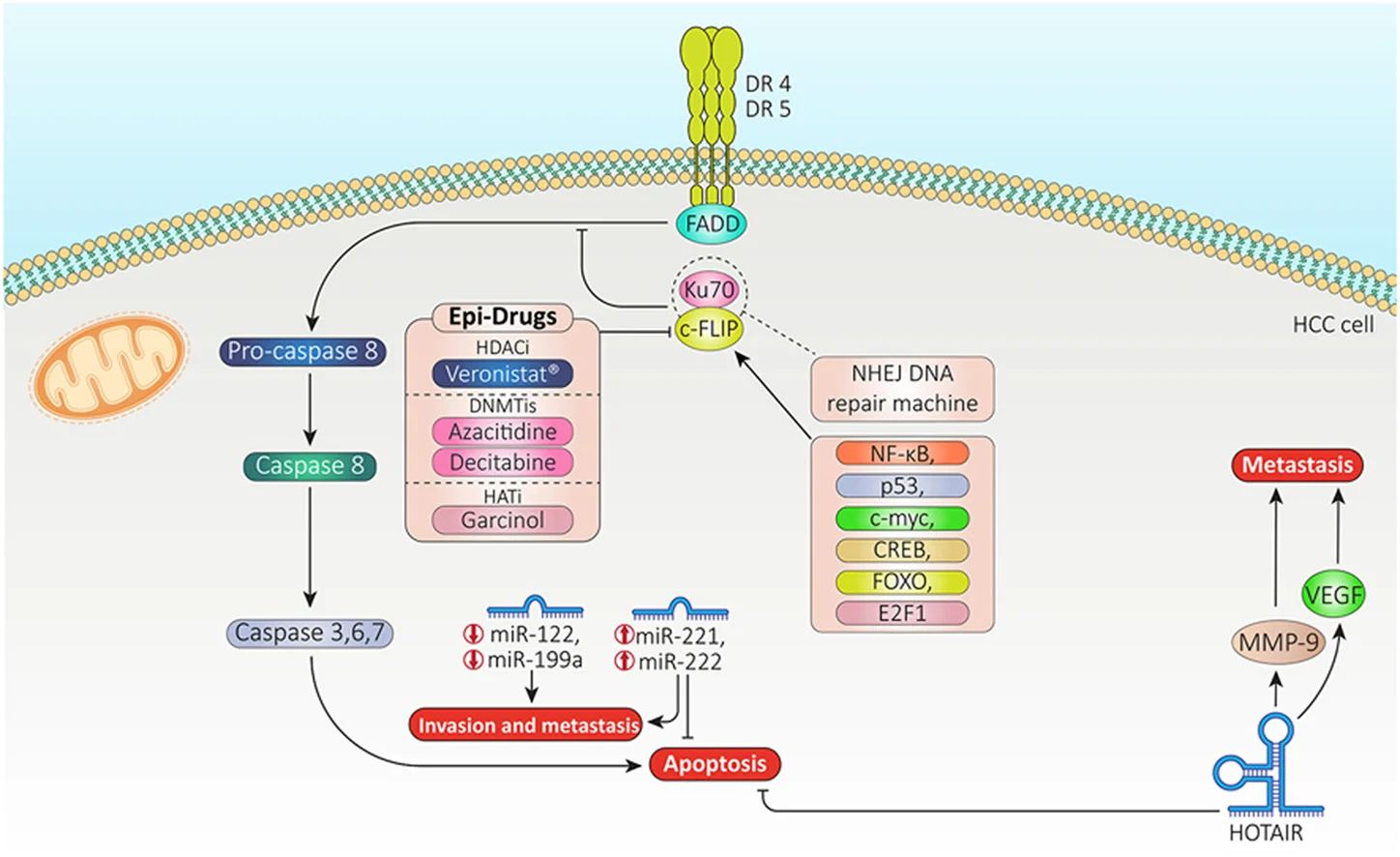
Apoptosis proceeds via intrinsic (mitochondrial) and extrinsic (death receptor) pathways. While BCL-2 family proteins primarily regulate the intrinsic pathway, c-FLIP is a key inhibitor of the extrinsic pathway, preventing caspase-8 activation at the death-inducing signaling complex (DISC).
Ku70, best known for its role in DNA repair via non-homologous end-joining, also functions in the cytoplasm as a stabilizer of c-FLIP. When bound together, the c-FLIP/Ku70 complex:
- Protects c-FLIP from ubiquitin-mediated degradation
- Sustains DISC inhibition
- Confers resistance to death receptor signaling in cancer cells
Crucially, Ku70’s affinity for c-FLIP is regulated by acetylation status. Deacetylated Ku70 binds and stabilizes c-FLIP, whereas acetylated Ku70 releases it, allowing c-FLIP degradation and reactivation of apoptotic signaling.
Role of Epigenetic Drugs
Histone deacetylase inhibitors (HDACis) act beyond chromatin remodeling. By increasing acetylation of non-histone proteins such as Ku70, HDACis:
- Disrupt the c-FLIP/Ku70 complex
- Promote c-FLIP ubiquitination and degradation
- Restore death receptor–mediated apoptosis in HCC cells
This non-genomic effect provides a mechanistic explanation for how epi-drugs can sensitize HCC cells to apoptosis, even in tumors resistant to conventional therapies.
c-FLIP/Ku70
Therapeutic Implications and Combinations
The review highlights the potential of combination strategies, particularly:
- HDAC inhibitors + tyrosine kinase inhibitors (e.g., sorafenib)
- HDAC inhibitors + death receptor ligands or TRAIL-based approaches
Importantly, the interplay between apoptosis and autophagy is emphasized. While autophagy may serve as a protective mechanism in HCC—contributing to resistance against TKIs—HDAC inhibition can modulate both pathways. Depending on context, combining HDACis with autophagy inhibitors may further enhance tumor cell death.
Biomarker Potential
Two molecular features emerge as promising diagnostic and prognostic biomarkers:
- Ku70 acetylation status
- c-FLIP ubiquitination levels
Assessing these parameters could help identify patients most likely to benefit from epigenetic-based therapies and guide treatment personalization in HCC.
Insights
This review reframes apoptosis resistance in HCC as a protein-interaction–driven and epigenetically regulated process, rather than a purely genetic defect. Targeting the c-FLIP/Ku70 complex represents a shift toward mechanism-based precision therapy, exploiting reversible epigenetic modifications to re-enable programmed cell death.
Key Takeaway Messages
- The c-FLIP/Ku70 complex is a critical mediator of apoptosis resistance in HCC.
- HDAC inhibitors can dissociate this complex via Ku70 acetylation, restoring extrinsic apoptosis.
- Combination regimens incorporating epi-drugs may overcome resistance to standard HCC therapies.
- Ku70 acetylation and c-FLIP ubiquitination hold promise as biomarkers for prognosis and treatment selection.
Conclusion
Targeting anti-apoptotic protein complexes through epigenetic modulation offers a rational and innovative strategy for HCC treatment. By disrupting the c-FLIP/Ku70 interaction, HDAC inhibitors can re-sensitize cancer cells to apoptosis and potentially improve outcomes when used alone or in combination regimens. As our understanding of epigenetic control over cell death deepens, this axis stands out as a fertile ground for translational research and future clinical development in hepatocellular carcinoma.
Read all article here