Immunotherapy is rapidly transforming the management of bladder cancer, expanding beyond metastatic disease into neoadjuvant, adjuvant, perioperative, and bladder-preserving settings. Immune checkpoint inhibitors targeting the PD-1/PD-L1 axis are now being integrated with chemotherapy, radiotherapy, antibody–drug conjugates, intravesical therapy, and novel immune-modulating strategies to improve outcomes across all disease stages.
Current clinical trials explore immunotherapy-driven approaches in both muscle-invasive and high-risk non–muscle-invasive bladder cancer, particularly for patients who are cisplatin-ineligible or who fail BCG therapy. These studies aim not only to enhance pathologic response and survival but also to preserve bladder function and reduce treatment-related morbidity.
Together, these efforts reflect a shift toward biology-driven, multimodal immunotherapy strategies, supported by biomarker analyses such as ctDNA, immune profiling, and tumor genomics, to personalize treatment and refine patient selection in bladder cancer.
Bladder Cancer: Symptoms ,Causes, Stages, Diagnosis and Treatment
Perioperative Immunotherapy With Sacituzumab Govitecan in Muscle-Invasive Bladder Cancer
This Phase II study explores a novel perioperative strategy for patients with muscle-invasive bladder cancer (MIBC) who are ineligible for or decline cisplatin-based chemotherapy—a group with limited standard options. The trial evaluates a triplet combination of sacituzumab govitecan, zimberelimab (PD-1 inhibitor), and domvanalimab (anti-TIGIT antibody), aiming to improve tumor eradication before surgery and reduce relapse after cystectomy.
Eligible patients with cT2–T4a, cN0–1, M0 urothelial carcinoma receive three cycles of the triplet regimen every three weeks prior to radical cystectomy. The primary endpoint is pathologic complete response (pCR) at surgery. Patients who do not achieve pCR—or who remain ctDNA-positive despite pCR—continue into an adjuvant phase with 12 additional cycles of zimberelimab and domvanalimab, targeting minimal residual disease and early molecular relapse.
To ensure safety, the study is conducted in sequential stages. An initial safety run-in evaluates the tolerability of sacituzumab govitecan plus zimberelimab in the first eight patients. Upon confirmation by an independent safety committee, the trial proceeds to test the full triplet combination.
This study represents an important shift in MIBC management, integrating an antibody–drug conjugate with dual immune checkpoint modulation in the perioperative setting. By offering a chemotherapy-free approach for cisplatin-ineligible patients, it aims to expand curative-intent options while leveraging immune-mediated tumor control both before and after surgery.
Concurrent Adjuvant Immunotherapy and Radiotherapy in Bladder Cancer
This Phase I study from the Abramson Cancer Center at Penn Medicine investigates the safety of combining adjuvant immune checkpoint inhibition with pelvic radiation therapy in patients with high-risk urothelial bladder cancer following radical cystectomy.
The trial focuses on patients who have already undergone radical cystectomy and pelvic lymph node dissection and are receiving standard adjuvant checkpoint inhibitor therapy, but remain at high risk of recurrence due to pathologic stage T3 or higher disease. Rather than testing new drugs, the investigational aspect of this study lies in the concurrent delivery of immunotherapy and radiation, a strategy that may enhance local tumor control but carries potential toxicity concerns.
Radiation therapy is initiated 12–18 weeks after the start of adjuvant immunotherapy and is delivered over approximately six weeks to the pelvic region. The primary objective is to define the acute safety profile of this combined approach, with particular attention to grade 3 or higher pelvic radiation–related toxicities, assessed using CTCAE v5.0 criteria. The regimen will be considered safe if the probability of severe pelvic toxicity remains below 20%.
Eligible patients must have no clinical or radiographic evidence of residual or recurrent disease at study entry, including those with microscopically positive surgical margins but no gross disease. A wide range of urothelial variant histologies is permitted, reflecting the real-world heterogeneity of bladder cancer. Patients with prior pelvic radiation, inflammatory bowel disease, orthotopic neobladders, or significant gastrointestinal complications are excluded to minimize overlapping toxicity risks.
This trial addresses an important unanswered question in post-cystectomy management: whether immunotherapy and radiation can be safely integrated in the adjuvant setting. If feasible, this approach could pave the way for future studies evaluating not only safety but also local control and survival benefits in high-risk bladder cancer.
Personalized mRNA Vaccine Plus BCG for High-Risk Non–Muscle-Invasive Bladder Cancer
This Phase II, open-label, randomized study evaluates whether adding intismeran autogene (V940)—a personalized mRNA cancer vaccine—to standard BCG therapy can improve outcomes in patients with high-risk non–muscle-invasive bladder cancer (HR NMIBC).
HR NMIBC, including carcinoma in situ (CIS), carries a high risk of recurrence and progression despite standard treatment with TURBT followed by intravesical BCG. Because a significant proportion of patients do not achieve durable responses with BCG alone, this study tests whether individualized immune priming with V940 can enhance and sustain antitumor immunity.
After recent TURBT, eligible patients are randomized to receive either:
- V940 in combination with BCG, or
- BCG alone (standard of care)
- V940 is a tumor-specific mRNA vaccine, designed using each patient’s tumor neoantigens to stimulate a targeted immune response against their cancer.
Study Population
The trial includes two biologically and clinically distinct cohorts:
- Cohort A: BCG-naïve patients with high-grade Ta, T1, and/or CIS bladder cancer
- Cohort B: Patients with CIS (± papillary disease) who are ineligible for or decline intravesical therapy, including selected BCG-exposed patients
Patients with muscle-invasive or metastatic disease are excluded.
Objectives
The primary goal is to determine whether V940 plus BCG improves cancer-free survival, defined as longer time without recurrence, progression, or worsening disease, compared with BCG alone. Safety, tolerability, and immune-related outcomes are also closely monitored.
Intracavitary Cryoablation Combined With Immunotherapy for Bladder Preservation
This prospective, single-center clinical study from Changhai Hospital explores an innovative bladder-preserving strategy for patients with high-risk non–muscle-invasive bladder cancer (NMIBC) who wish to avoid radical cystectomy. The trial integrates precise endoluminal cryoablation with immunotherapy and low-dose chemotherapy, aiming to improve tumor control while preserving bladder function and quality of life.
The experimental approach combines standard TURBT with a novel liquid nitrogen balloon cryoablation system, applied directly to the tumor base under endoscopic guidance. By inducing immunogenic tumor cell death, cryoablation is expected to enhance responsiveness to subsequent anti–PD-1 immunotherapy. Patients then receive adjuvant PD-1 blockade (such as camrelizumab or tislelizumab) together with metronomic gemcitabine–cisplatin chemotherapy, forming the so-called TECIC model.
A total of 180 patients with biopsy- or TURBT-confirmed high-risk NMIBC are enrolled. Those choosing bladder preservation receive the TECIC regimen, while the control group undergoes radical cystectomy with urinary diversion, reflecting current standard practice. The primary endpoint is 5-year overall survival, with secondary outcomes including progression-free survival, intravesical recurrence, and patient-reported quality of life.
Beyond clinical efficacy, the study also investigates biological correlates of response, including changes in local immune infiltration and systemic immune markers before and after treatment. By directly comparing a multimodal bladder-sparing approach against cystectomy, this trial addresses a critical unmet need in NMIBC—whether effective oncologic control can be achieved without sacrificing the bladder.
Concurrent Adjuvant Immunotherapy and Radiotherapy After Cystectomy
This Phase I study from the Abramson Cancer Center at Penn Medicine evaluates the safety of combining adjuvant immune checkpoint inhibition with pelvic radiotherapy in patients with high-risk urothelial bladder cancerfollowing radical cystectomy.
Eligible patients have pathologic T3 or higher disease (any N, M0) after cystectomy and lymph node dissection and are already receiving standard adjuvant checkpoint inhibitor therapy. The investigational aspect of the trial is the concurrent delivery of immunotherapy and external beam radiation, rather than the sequential approach typically used in clinical practice.
Radiotherapy begins 12–18 weeks after starting adjuvant immunotherapy and is delivered over approximately six weeks. The primary objective is to establish acute safety, specifically the rate of grade ≥3 pelvic radiation–related toxicities, assessed using CTCAE v5.0. The regimen will be considered safe if the probability of severe acute toxicity remains below a predefined threshold.
This study addresses an important clinical question in bladder cancer management: whether postoperative radiation can be safely combined with immune checkpoint blockade in patients at high risk of recurrence. If feasible, this strategy could strengthen local disease control in the adjuvant setting without compromising tolerability, laying the groundwork for future efficacy-focused trials.
Perioperative Sacituzumab Govitecan–Based Immunotherapy in Muscle-Invasive Bladder Cancer
This open-label, multicenter Phase II trial evaluates a novel perioperative antibody–drug conjugate and immunotherapy combination for patients with muscle-invasive bladder cancer (MIBC) who are ineligible for or decline cisplatin-based neoadjuvant chemotherapy.
Eligible patients with cT2–T4a, cN0–1, cM0 urothelial carcinoma planned for cystectomy receive three neoadjuvant cycles of sacituzumab govitecan, combined with zimberelimab (anti–PD-1) and domvanalimab (anti–TIGIT), administered every three weeks prior to surgery. The primary endpoint is pathologic complete response (pCR) at cystectomy.
Patients who do not achieve a pCR—or who achieve pCR but remain ctDNA-positive—continue into an adjuvant phasewith 12 additional cycles of zimberelimab plus domvanalimab, aiming to eradicate residual micrometastatic disease and reduce recurrence risk.To ensure patient safety, the study incorporates a stepwise safety run-in design. An initial cohort of eight patients receives the doublet (sacituzumab govitecan + zimberelimab) to confirm tolerability. Following external safety committee review, the study proceeds to a second run-in cohort evaluating the full triplet regimen.
This trial addresses a key unmet need in bladder cancer by exploring a chemo-free, immunotherapy-driven perioperative strategy for cisplatin-ineligible MIBC. By combining TROP-2–targeted cytotoxic delivery with dual immune checkpoint blockade, the study aims to improve pathologic response rates and long-term outcomes in a population with limited standard treatment options.
Perioperative Sacituzumab Govitecan–Based Immunotherapy in Muscle-Invasive Bladder Cancer
This open-label, multicenter Phase II study evaluates a perioperative, chemotherapy-free immunotherapy strategyfor patients with muscle-invasive bladder cancer (MIBC) who are ineligible for or decline cisplatin-based neoadjuvant chemotherapy.
Eligible patients with cT2–T4a, cN0–1, cM0 urothelial carcinoma receive three neoadjuvant cycles of sacituzumab govitecan combined with zimberelimab (anti–PD-1) and domvanalimab (anti–TIGIT) prior to radical cystectomy. The primary endpoint is pathologic complete response (pCR) at surgery.
Patients who fail to achieve pCR—or who achieve pCR but remain ctDNA-positive—continue into an adjuvant phasewith 12 additional cycles of zimberelimab plus domvanalimab, aiming to eliminate residual micrometastatic disease and reduce recurrence risk.
To prioritize safety, the study follows a stepwise run-in design. An initial cohort of eight patients receives sacituzumab govitecan plus zimberelimab to confirm tolerability. Upon approval by an external safety committee, enrollment proceeds to the full triplet combination.
This trial explores a novel perioperative approach integrating a TROP-2–directed antibody–drug conjugate with dual immune checkpoint blockade, addressing a major unmet need for effective neoadjuvant options in cisplatin-ineligible MIBC.
Lurbinectedin With or Without Avelumab in Small Cell Carcinoma of the Bladder
This Phase II, multicenter, open-label study led by the National Cancer Institute is addressing a major unmet need in small cell carcinoma of the bladder (SCCB) and other high-grade neuroendocrine tumors (HGNETs) of the urinary tract—rare, highly aggressive cancers with a median survival of roughly one year and very limited treatment options after first-line therapy.
Historically, SCCB has been treated using platinum–etoposide regimens borrowed from small cell lung cancer, which often induce rapid responses but are rarely durable. There is little prospective evidence to guide treatment once the disease progresses, particularly regarding the role of immunotherapy.
This trial evaluates lurbinectedin, a transcriptional inhibitor already approved in relapsed small cell lung cancer, with or without the PD-L1 inhibitor avelumab. All participants receive lurbinectedin, while those without prior immune checkpoint inhibitor exposure also receive avelumab.
Study Design and Treatment
Population: Adults with metastatic, measurable SCCB or other urinary tract HGNETs that have progressed after, are ineligible for, or declined platinum–etoposide chemotherapy
Cohorts:
- Lurbinectedin alone (prior ICI exposure)
- Lurbinectedin + avelumab (ICI-naïve)
Dosing:
- Lurbinectedin 3.2 mg/m² IV every 21 days
- Avelumab 800 mg IV every 21 days (when applicable)
Treatment continues until progression or unacceptable toxicity, with long-term follow-up up to 10 years.
Objectives
The primary goal is to assess objective response rate (ORR) to lurbinectedin alone or in combination with avelumab. Secondary aims include durability of response, safety, and long-term outcomes in this rare disease population.
Vitamin B3 to Protect and Enhance Immunotherapy in Bladder Cancer
This Phase Ib clinical study explores whether Vitamin B3 (niacin) can help preserve or enhance the effectiveness of immunotherapy in patients with muscle-invasive bladder cancer who have recently been exposed to bactericidal antibiotics. The trial is based on the growing recognition that antibiotics, while often unavoidable, may impair antitumor immune responses and reduce the benefit of immune checkpoint inhibitors by disrupting immune–microbiome interactions.
The study enrolls adults with cT2–T4aN0M0 urothelial carcinoma of the bladder who have residual disease after TURBT and who required oral or intravenous antibiotics for a documented bacterial infection within the month preceding treatment. All patients receive standard neoadjuvant chemo-immunotherapy consisting of tislelizumab combined with gemcitabine and cisplatin, administered in 21-day cycles for up to six cycles, followed by tislelizumab maintenance therapy. In addition to this backbone regimen, patients receive daily oral Vitamin B3 supplementation at one of two predefined doses, starting after completion of antibiotic therapy.
The primary aim of the study is to define the safety and dose-limiting toxicity of Vitamin B3 when given alongside chemo-immunotherapy in this specific, antibiotic-exposed population. Secondary objectives focus on antitumor efficacy, including pathologic complete response, pathologic downstaging at cystectomy, and overall treatment-related safety, including surgical complications. Exploratory analyses will examine immune and molecular biomarkers in tumor tissue, blood, and urine, seeking to understand how Vitamin B3 may influence immune activation, tumor biology, and treatment response.
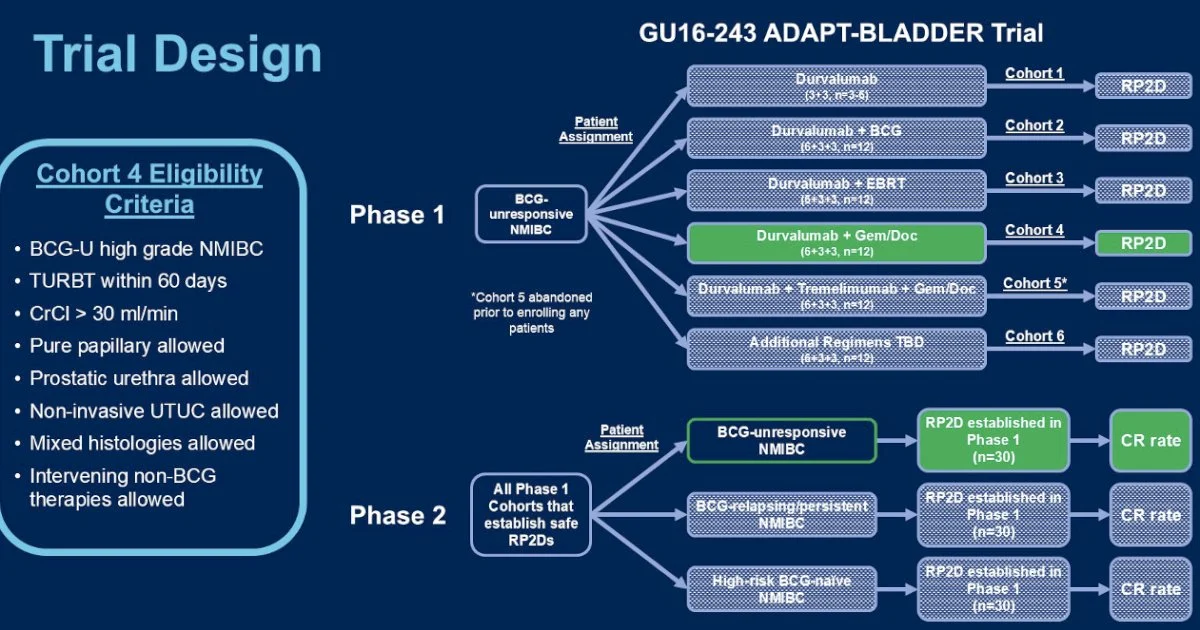
ADAPT-BLADDER: A Phase 1/2 Platform Trial of Modern Immunotherapy-Based Bladder Preservation in High-Risk Non-Muscle Invasive Bladder Cancer
ADAPT-BLADDER is a Phase 1/2 clinical trial designed to explore modern immunotherapy–based bladder-preserving strategies for patients with high-risk non-muscle invasive urothelial carcinoma (NMIBC), including those who are BCG-unresponsive, BCG-relapsing, BCG-persistent, or high-risk BCG-naïve.
The study is built as a stepwise, safety-first platform trial. It begins with durvalumab monotherapy, which serves as the initial safety cohort. If durvalumab alone demonstrates acceptable tolerability, the trial expands into multiple parallel cohorts that evaluate durvalumab in combination with other bladder-preserving treatments, including BCG, intravesical chemotherapy (gemcitabine and docetaxel), dual immune checkpoint blockade (durvalumab plus tremelimumab), and external beam radiotherapy (EBRT). Because of the long half-life of antibody therapies, durvalumab dosing is fixed across cohorts, while BCG dose adjustments are permitted if toxicity thresholds are exceeded.
The trial enrolls adults with Ta, T1, or carcinoma in situ (CIS) confirmed on recent TURBT, good performance status, and no evidence of muscle-invasive or metastatic disease. Patients are stratified by prior BCG exposure, allowing the study to address distinct clinical scenarios—from patients who have failed adequate BCG therapy to those who are BCG-naïve but harbor high-risk disease. In Phase 1, the primary goal is to establish dose-limiting toxicity and regimen feasibility. In Phase 2, selected regimens expand into disease-specific cohorts to evaluate antitumor activity and durability of bladder preservation.
Importantly, ADAPT-BLADDER reflects a modern, flexible trial design that mirrors real-world NMIBC complexity. Rather than testing a single regimen, it functions as a therapeutic platform, allowing promising immunotherapy-based combinations to advance while unsafe or ineffective strategies are halted early. The overarching aim is to identify effective bladder-sparing alternatives for patients who otherwise face radical cystectomy due to BCG failure or high-risk disease biology.