Gastrointestinal stromal tumors (GISTs) are driven primarily by oncogenic KIT mutations, and breakthroughs such as imatinib, sunitinib, and ripretinib have transformed the disease’s management. Yet resistance emerges almost universally, driven by secondary KIT mutations—particularly those occurring in the activation loop at exon 17/18, a region historically difficult to inhibit. Patients whose tumors harbor these mutations often progress rapidly, with few effective therapeutic options beyond later-line ripretinib.
The PEAK Phase 3 trial of bezuclastinib, a highly selective, next-generation KIT inhibitor from Cogent Biosciences, addresses this critical unmet need. The company’s announcement of positive topline results positions bezuclastinib as a potentially transformative therapy for patients with KIT activation-loop–mutant GIST.

Read About Gastrointestinal Stromal Tumor on OncoDaily
A Next-Generation KIT Inhibitor Engineered for Activation Loop Resistance
Bezuclastinib is designed with a singular purpose: potently and selectively inhibit KIT activation-loop mutations—particularly those in exons 17 and 18, which are historically resistant to nearly all approved TKIs. The drug’s molecular design prioritizes:
- High selectivity for KIT over PDGFRα, VEGFR, and other kinases, minimizing off-target toxicity
- Potent blockade of key resistance mutations, including KIT D816V and analogous activation loop substitutions
- Deep suppression of MAPK and PI3K/AKT signaling, the pathways that fuel proliferation and survival in resistant GIST clones
Preclinical models showed strong tumor regression and durable pathway inhibition. Early-phase clinical trials demonstrated activity even in heavily pretreated patients, providing the rationale for advancing bezuclastinib into the global PEAK Phase 3 study.
The PEAK Phase 3 Trial: Designed to Overcome Resistance Mechanisms
PEAK is a randomized, global Phase 3 trial evaluating:
Bezuclastinib + sunitinib vs. Sunitinib monotherapy in patients with imatinib-resistant or intolerant GIST, with an enriched focus on tumors harboring KIT exon 17/18 activation-loop mutations—the population most likely to benefit from bezuclastinib’s mechanism of action.
Key endpoints included:
• Primary: Progression-free survival (PFS) by blinded independent review
• Secondary: Objective response rate (ORR), duration of response (DoR), overall survival (OS, immature), and safety
This is the first successful Phase 3 trial in second-line GIST in more than 20 years, confirming the unmet need and significance of the results.
PEAK Phase 3 Results: A Landmark Efficacy Breakthrough
Cogent Biosciences announced exceptionally positive topline data, exceeding expectations and validating bezuclastinib’s mechanistic promise.
Progression-Free Survival (Primary Endpoint)
- 16.5 months mPFS — bezuclastinib + sunitinib
- 9.2 months mPFS — sunitinib alone
- Hazard Ratio = 0.50 (95% CI: 0.39–0.65)
- p < 0.0001
This represents a 7.3-month improvement in mPFS and a 50% reduction in risk of progression or death.
Objective Response Rate (ORR)
- 46% ORR with the bezuclastinib combination
- 26% ORR with sunitinib monotherapy
- p < 0.0001
This is the highest ORR ever reported in imatinib-resistant GIST in a Phase 3 trial, demonstrating profound antitumor activity.
Durability and Treatment Exposure
Cogent projects that the mean duration of treatment for patients on the bezuclastinib arm will exceed 19 months, underscoring durable disease control.
Overall Survival
OS data remain immature and will be presented as they mature in 2026.
Why These Results Matter for GIST Treatment
While first-line imatinib continues to provide long-term benefit for many patients, resistance develops through clonal evolution, most commonly involving mutations in KIT exons 13/14 (ATP-binding pocket) or exons 17/18 (activation loop). Sunitinib and regorafenib offer partial control of ATP-pocket mutations, but activation loop mutations remain highly resistant to almost all approved TKIs.
Bezuclastinib directly addresses this resistance mechanism. By selectively targeting the activation loop, it attacks the mutational drivers responsible for disease progression at a molecular level traditional TKIs cannot reach. The positive PEAK results therefore mark one of the most meaningful advancements in the sequential TKI strategy for GIST since ripretinib entered the landscape.
For clinicians, this means the potential for a more precise, biomarker-driven approach: identifying patients with KIT exon 17/18 mutations and offering them therapy specifically designed to neutralize the resistant clone.
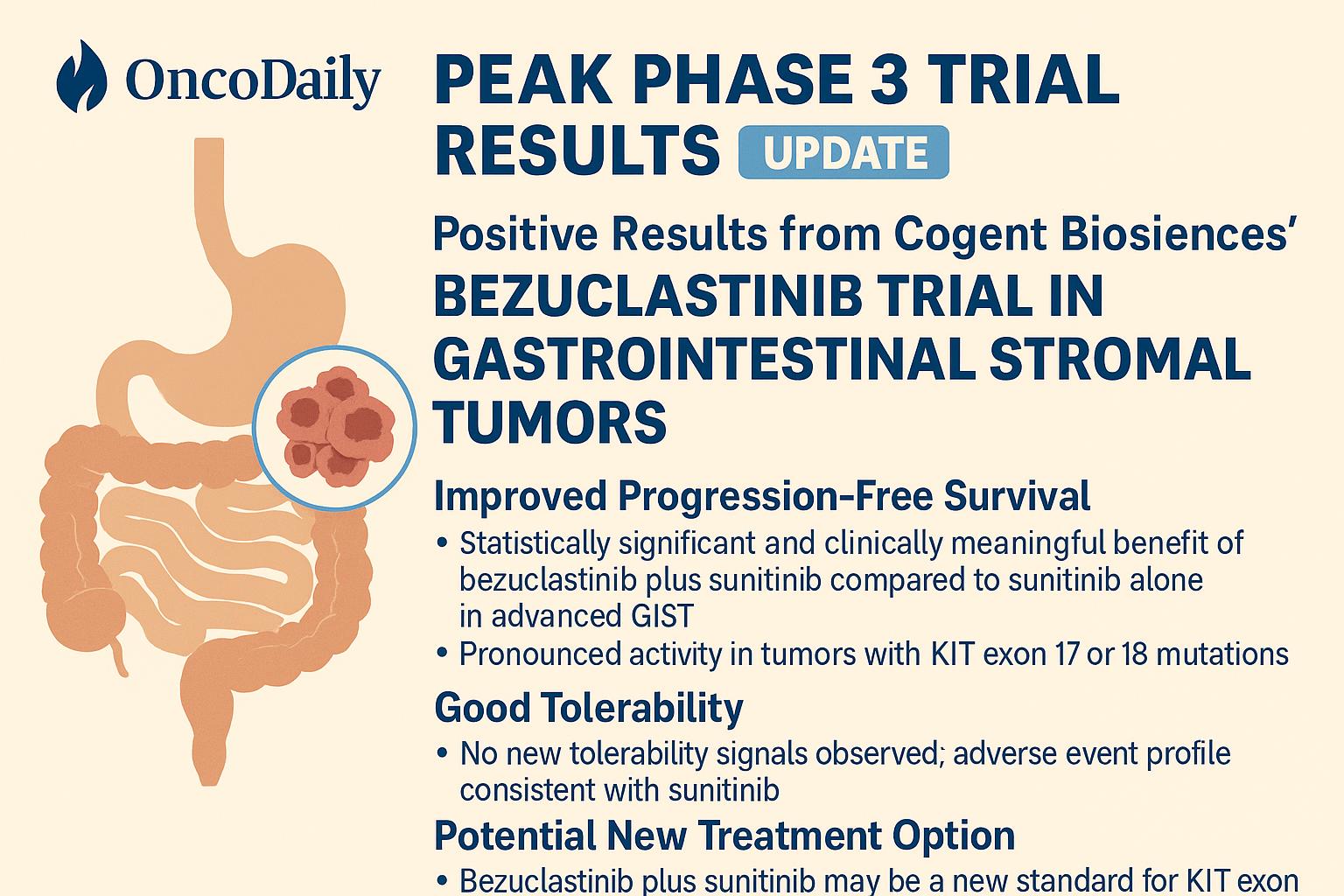
Safety Profile and Clinical Considerations
The combination of bezuclastinib + sunitinib was:
- Generally well tolerated
- Consistent with sunitinib’s known profile
- Not associated with any new or unexpected safety signals
Grade ≥3 events included (combo vs. sunitinib):
- Hypertension: 29.4% vs. 27.4%
- Neutropenia: 15.2% vs. 15.4%
- ALT/AST increased: 10.8% vs. 1.4%
- Anemia: 9.3% vs. 4.8%
- Diarrhea: 7.8% vs. 7.2%
Hepatic events were transient, manageable, and reversible, with no grade 4 elevations and only 1.5% treatment discontinuation due to ALT/AST increases.
This safety profile reinforces bezuclastinib’s selectivity advantage, enabling sustained dosing—a critical factor in the chronic management of GIST.
Implications for Clinical Practice and Future Development
If the PEAK results are supported by full data presentation and regulatory review, bezuclastinib may soon become the preferred treatment option for KIT exon 17/18 mutant GIST after imatinib failure. The drug’s specificity, tolerability, and clear clinical benefit position it to reshape the treatment sequence in a mutation-guided manner.
Beyond PEAK, bezuclastinib is also in development for systemic mastocytosis—another KIT activation loop–driven disease—illustrating the drug’s broad potential across KIT-dependent malignancies. Its success in GIST underscores the value of highly selective inhibitors capable of countering resistance mechanisms that have limited the durability of existing TKIs.
Conclusion
The positive results from the PEAK Phase 3 trial highlight bezuclastinib as a promising and potentially practice-changing therapy for patients with KIT exon 17/18 mutant gastrointestinal stromal tumors. By addressing one of the most formidable resistance mechanisms in GIST biology, bezuclastinib fills a long-standing therapeutic gap and offers clinicians a new pathway toward sustained disease control in a population with limited options.
Pending further analyses and regulatory submissions, bezuclastinib has the potential to become a new standard of care for this genetically defined subset of GIST, bringing precision oncology one step closer to everyday practice.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


