Avutometinib and defactinib are two targeted therapies that have recently shown promise in treating recurrent low-grade serous ovarian cancer (LGSOC). Avutometinib, a mitogen-activated extracellular signal-regulated kinase (MEK) inhibitor, works by blocking the MEK pathway, which is frequently dysregulated in LGSOC. Defactinib, a focal adhesion kinase (FAK) inhibitor, targets a key adaptive resistance mechanism that compensates for the inhibition of MEK. LGSOC, a rare subtype of ovarian cancer, is known to present at a younger age and is less responsive to chemotherapy compared to high-grade serous ovarian cancer (HGSOC).
Although it accounts for less than 10% of all ovarian cancer cases, LGSOC is often driven by mutations in the mitogen-activated protein kinase (MAPK) pathway, particularly the KRAS mutation, found in about 30% of patients. Traditional treatment approaches for recurrent LGSOC have had limited success, with chemotherapy response rates ranging from 0% to 13%. Given the poor prognosis for these patients and the limited treatment options, this article examines the results of a phase II trial evaluating the combination of avutometinib and defactinib, which aims to provide a more effective treatment option for recurrent LGSOC, particularly for patients with KRAS mutations.
Background
Low-grade serous ovarian cancer (LGSOC) is a rare but distinct form of ovarian cancer, accounting for less than 10% of all new epithelial ovarian cancer cases. Compared to high-grade serous ovarian cancer (HGSOC), LGSOC generally presents at a younger age and shows reduced sensitivity to chemotherapy. LGSOC is often driven by mitogen-activated protein kinase (MAPK) mutations, most notably the Kirsten rat sarcoma virus homolog (KRAS) mutation, found in about 30% of patients. Patients with KRAS-mutant tumors tend to have better prognoses than those with KRAS wild-type tumors.
Conventional treatments for recurrent LGSOC have shown limited success, with chemotherapy response rates ranging from 0% to 13%. Recent studies indicate that mitogen-activated extracellular signal-regulated kinase (MEK) inhibitors have demonstrated improved response rates, but toxicity and discontinuation rates have hindered their efficacy. A promising approach involves combining MEK inhibition with focal adhesion kinase (FAK) inhibition to target adaptive resistance mechanisms. This study evaluates the combination of avutometinib, a novel MEK inhibitor, and defactinib, a FAK inhibitor, to assess efficacy and safety in patients with recurrent LGSOC.
Methods
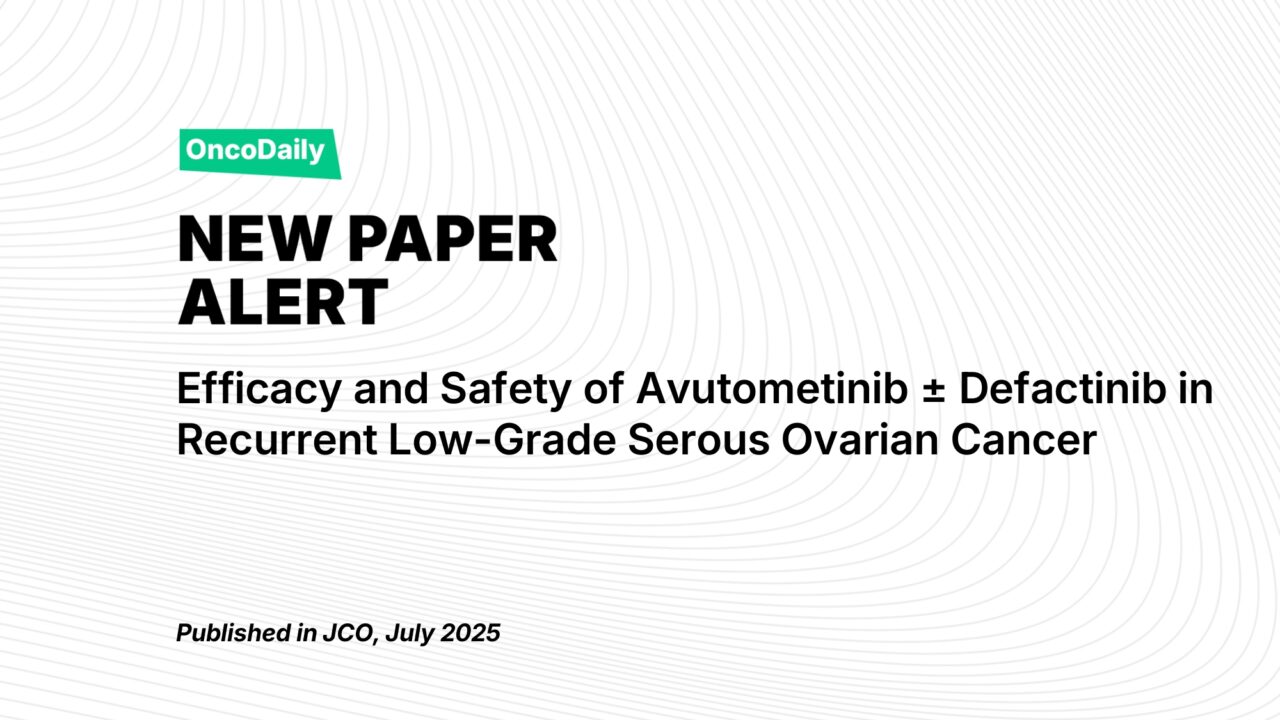
This phase II, multicenter, randomized, open-label trial aimed to compare the efficacy and safety of avutometinib alone versus its combination with defactinib in patients with recurrent LGSOC. The study included patients with histologically confirmed LGSOC who had measurable disease, and who had previously received at least one line of systemic therapy, including platinum-based chemotherapy. KRAS mutation status was assessed prior to enrollment. The trial was divided into four parts, with the primary endpoint being the objective response rate (ORR) according to RECIST v1.1, assessed by blinded independent central review (BICR). Secondary endpoints included duration of response (DOR), progression-free survival (PFS), and overall survival (OS), alongside the safety profile of the treatments.
Study Design
Patients were randomly assigned to receive avutometinib 4.0 mg twice a week or a combination of avutometinib 3.2 mg twice a week plus defactinib 200 mg twice daily for a 4-week cycle (3 weeks of treatment followed by 1 week of rest). The trial included a stratification by KRAS mutation status to ensure equal distribution of patients with KRAS-mutant and KRAS wild-type tumors. The study was conducted across several countries, including the United States, the United Kingdom, and European nations, with institutional review board approval at each site.
Results
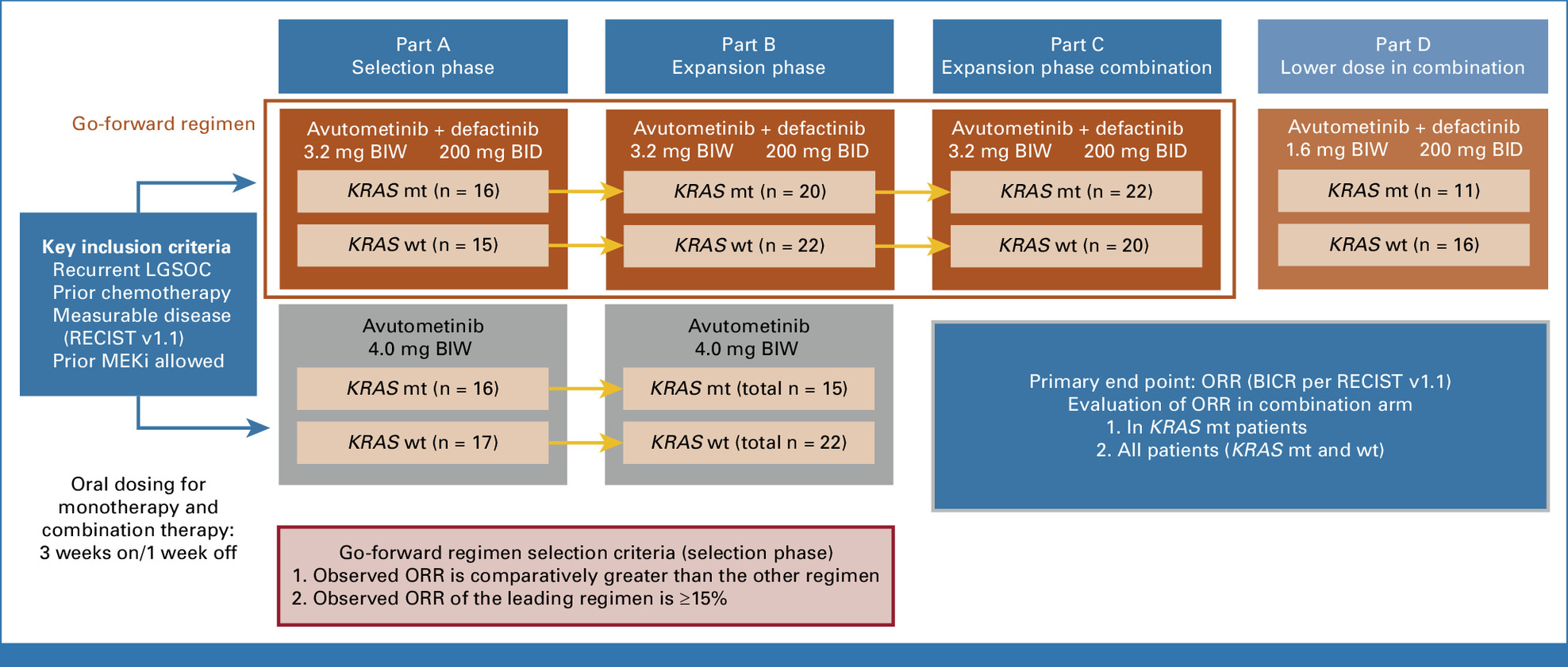
The phase II trial evaluated the efficacy and safety of the combination of avutometinib and defactinib in patients with recurrent low-grade serous ovarian cancer (LGSOC). A total of 185 patients were enrolled, with 115 receiving the combination treatment and 70 receiving avutometinib monotherapy. The study aimed to assess the primary endpoint of objective response rate (ORR), along with secondary endpoints such as duration of response (DOR) and progression-free survival (PFS). The data from the trial provided valuable insights into the efficacy of the treatment regimens, as well as the impact of KRAS mutation status on treatment response.
Combination Treatment (Avutometinib + Defactinib):
- ORR: 31% overall
- KRAS-mutant patients: 44% response rate
- KRAS wild-type patients: 17% response rate
- Median DOR: 31.1 months- 81% of responses maintained at 6 months, 72% of responses maintained at 12 months
- Median PFS: 12.9 months- KRAS-mutant patients: 22.0 months, KRAS wild-type patients: 12.8 months
- 82% of patients showed tumor shrinkage (reduction in target lesions)
Monotherapy (Avutometinib):
- ORR: 17% overall
- KRAS-mutant patients: 23% response rate
- KRAS wild-type patients: 13% response rate
Subgroup Analysis:
- Patients with 1-3 prior therapies: 37% ORR
- Patients with more than 3 prior therapies: 24% ORR
Key Findings
- The combination of avutometinib and defactinib resulted in an ORR of 31%, significantly higher than the 17% ORR seen with avutometinib monotherapy.
- KRAS-mutant patients showed a better treatment response, with an ORR of 44% and a median PFS of 22.0 months, compared to 17% and 12.8 months for KRAS wild-type patients, respectively.
- The median DOR for the combination treatment was 31.1 months, suggesting that responses were both durable and clinically meaningful.
- The combination regimen demonstrated manageable toxicity profiles, with 56% of patients requiring dose holds, and 10% discontinuing treatment due to adverse events (AEs).
- Elevated creatine phosphokinase (CPK) was the most common laboratory abnormality (60% of patients), although most were asymptomatic and manageable with dose modifications.
Key Takeaway Messages
- The combination of avutometinib and defactinib showed clinically significant efficacy in recurrent LGSOC, especially in patients with KRAS mutations.
- KRAS mutation status plays a crucial role in determining treatment response, with KRAS-mutant patients having significantly better outcomes.
- The combination regimen offers a promising new option for patients with recurrent LGSOC, a population with limited treatment alternatives.
- The treatment’s safety profile was acceptable, with manageable AEs, most of which could be addressed through dose adjustments, allowing prolonged exposure to therapy.
- The study’s findings support avutometinib plus defactinib as a potential new standard of care for recurrent LGSOC, although further studies with comparator arms are needed to confirm these results.
Conclusion
This phase II trial demonstrates the potential of the combination of avutometinib and defactinib as an effective treatment for recurrent LGSOC, particularly in patients with KRAS mutations. The higher ORR and prolonged PFS observed in the combination group compared to avutometinib monotherapy provide evidence that this regimen may significantly improve outcomes for patients with this rare and difficult-to-treat cancer. Importantly, the safety profile was manageable, with most adverse events being reversible through dose holds or adjustments.
These results suggest that avutometinib combined with defactinib could become a new standard of care for recurrent LGSOC, especially for patients who are KRAS-mutant. However, further studies, including phase III trials with active comparator arms, are required to confirm the long-term benefits of this treatment and to better understand its role in the broader clinical landscape of LGSOC management.
You can read the full article here.