On May 8, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to the combination of avutometinib and defactinib (Avmapki Fakzynja Co-pack, Verastem, Inc.) for the treatment of adult patients with KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC) who have received prior systemic therapy.
What is the Rationale for FDA approval of Avutometinib and Defactinib in KRAS-Mutated Recurrent LGSOC?
The RAMP 201 trial (NCT04625270) is an adaptive, open-label, multicenter study evaluating the efficacy and safety of the combination of avutometinib, an oral RAF/MEK clamp, and defactinib, a selective FAK inhibitor, in patients with recurrent low-grade serous ovarian cancer. The trial included patients whose disease had progressed despite prior treatments, including chemotherapy, MEK inhibitors, and bevacizumab. The updated data, with a cutoff of June 30, 2024, show robust overall response rates and progression-free survival outcomes, particularly in the KRAS mutant subgroup.
PAMP 201 Trial Design and Methods
The RAMP 201 trial enrolled 109 patients with recurrent LGSOC who were treated with the combination of avutometinib and defactinib. The primary analysis of the trial focused on the overall response rate (ORR) and progression-free survival (PFS), with the data showing significant clinical benefit for patients, particularly those with KRAS mutations.
The primary endpoints included ORR and median PFS. The trial’s secondary endpoints included disease control rate (DCR) and the duration of response (DOR).
Results of PAMP 201: Notable Efficacy in KRAS Mutant Subgroup
As of June 2024, the data revealed a confirmed ORR of 31% across the entire evaluable patient population. For patients with KRAS mutations, the ORR was significantly higher at 44%, whereas those with KRAS wild-type LGSOC had an ORR of 17%. These results underscore the therapeutic benefit of the combination for KRAS-mutant patients.
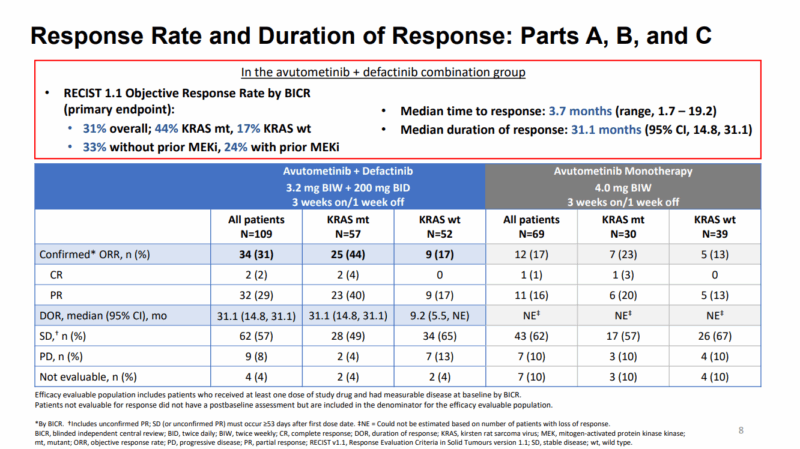
The duration of response (DOR) was also impressive, with a median DOR of 31.1 months in the overall population. For the KRAS-mutant subgroup, the DOR remained at 31.1 months, while the KRAS wild-type subgroup saw a shorter DOR of 9.2 months.
In terms of progression-free survival (PFS), the median PFS was 12.9 months for all patients, with a remarkable 22 months for those with KRAS mutations. The disease control rate (DCR) at 6 months or more was 61% in the overall population, with a higher rate of 70% in the KRAS-mutant group, compared to 50% in the KRAS wild-type group.
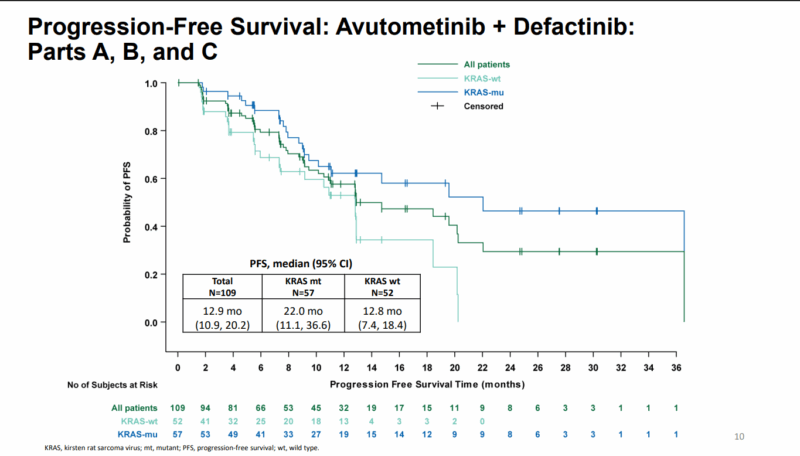
Source: IGCS | 2024 Annual Global Meeting|Dublin
Safety Profile
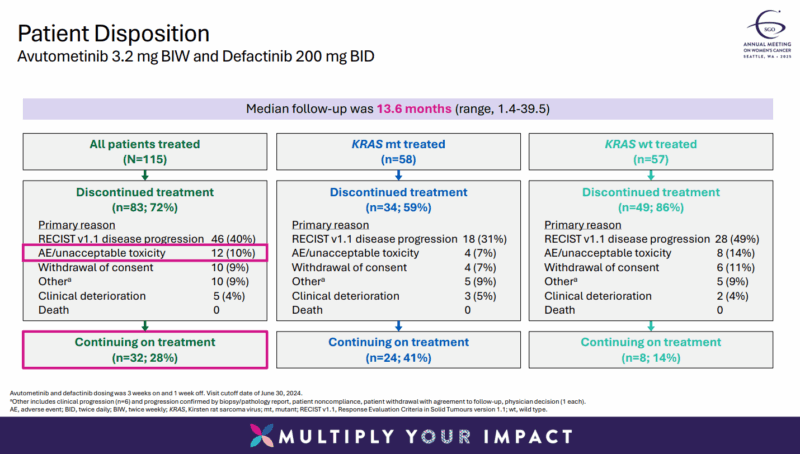
The combination of avutometinib and defactinib was generally well tolerated, with a 10% discontinuation rate due to adverse events. The most common treatment-related side effects included nausea (67%), diarrhea (58%), and increased blood creatine phosphokinase levels (60%), with grade 3 or higher adverse events occurring in a small percentage of patients.
Dosage and Administration
The recommended dose of avutometinib is 3.2 mg (four 0.8 mg capsules) taken orally twice weekly for the first three weeks of each 4-week cycle, until disease progression or unacceptable toxicity. The recommended dose of defactinib is 200 mg (one tablet) taken orally twice daily during the first three weeks of each 4-week cycle, also until disease progression or unacceptable toxicity.
In conclusion this FDA approval marks an important milestone in the treatment of KRAS-mutated recurrent low-grade serous ovarian cancer, offering a promising new therapeutic option for patients who have previously been treated with chemotherapy and other systemic therapies.
Here you can read about trials results.
How does Avutometinib and Defactinib work?
Avutometinib is a MEK inhibitor (mitogen-activated protein kinase kinase inhibitor). MEK is part of the RAS-RAF-MEK-ERK signaling pathway, which is involved in cell growth and survival. When this pathway is activated abnormally, it can contribute to cancer development and progression. Avutometinib works by selectively inhibiting MEK1 and MEK2, two enzymes that play a crucial role in this signaling pathway. By blocking MEK, the drug effectively shuts down the signals that drive the growth and survival of cancer cells, thus slowing down or halting tumor progression.
Defactinib is a FAK (focal adhesion kinase) inhibitor, a protein that is involved in the regulation of cell movement, survival, and attachment to the extracellular matrix. In cancer cells, FAK is often overactive, promoting tumor growth and metastasis (the spread of cancer to other parts of the body). By inhibiting FAK, defactinib disrupts these processes, potentially preventing cancer cells from growing and spreading. It also enhances the immune system’s ability to target and kill cancer cells, making it a valuable therapeutic option in cancers that are difficult to treat with conventional therapies.
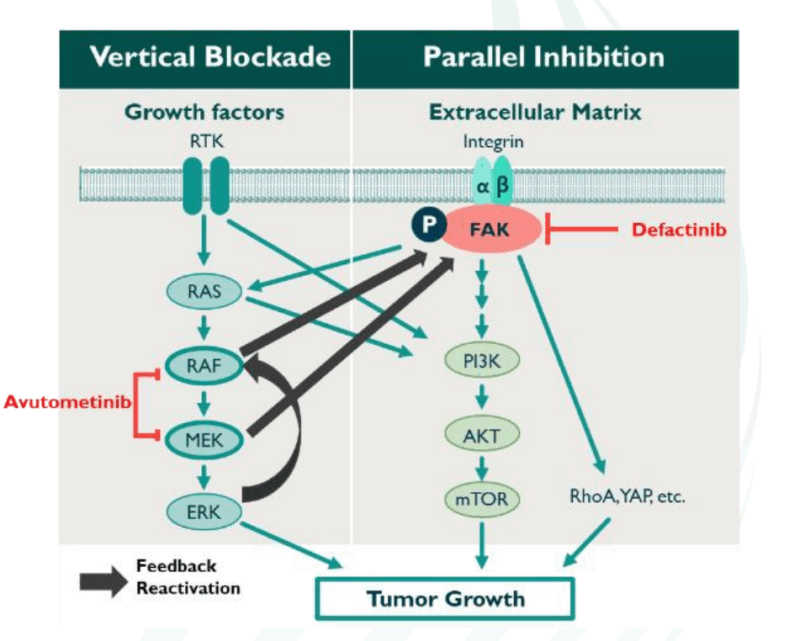
Source: IGCS | 2024 Annual Global Meeting|Dublin
Both of these drugs are designed to target specific molecular pathways that are critical for cancer cell survival and spread. By interfering with these pathways, they aim to slow down the progression of cancer, reduce the size of tumors, and improve overall treatment outcomes for patients with certain types of cancer.
Reactions to FDA Approval of Avutometinib and Defactinib for KRAS-Mutant LGSOC
The recent FDA approval of avutometinib and defactinib for KRAS-mutant low-grade serous ovarian cancer (LGSOC) has drawn attention across social media platforms, especially LinkedIn, where experts shared their perspectives.
Oliver Maracic, an investor, posted a detailed breakdown of response rates from the RAMP 201 trial. He noted that in KRAS-mutant patients, response rates were higher in those with no prior MEK inhibitor (47%) or bevacizumab exposure (53%). Patients who had received fewer prior therapies also showed better outcomes. His post highlighted how patient history can influence treatment response and reinforced the promise of this new combination therapy.
Find more info here.
Jonathan Pachter, Chief Scientific Officer at Verastem Oncology, expressed his excitement about the approval, calling it the first targeted therapy for patients with KRAS-mutant LGSOC. He shared his gratitude for the Verastem team and pride in helping bring a new option to this underserved patient population.
What is Ovarian Cancer?
Ovarian cancer is often called the “silent killer” because its symptoms are subtle and can be mistaken for other conditions, making early detection challenging. In 2025 according to American Cancer Society estimated that 20,890 women in the U.S. will be diagnosed, and 12,730 will die from the disease. The lifetime risk of developing ovarian cancer is 1 in 91, and 1 in 143 will die from it.
The symptoms of ovarian cancer often appear gradually and can be mistaken for other conditions. Persistent bloating, pelvic pain, frequent urination, and a feeling of fullness are common signs that women might experience, but they often worsen as the cancer advances. These symptoms, unfortunately, tend to be overlooked or misdiagnosed, which is why many women don’t receive a diagnosis until the cancer has progressed to a more advanced stage.
Diagnosing ovarian cancer is a challenge due to its subtle symptoms and lack of reliable early detection methods. Doctors often use pelvic exams, ultrasounds, CT or MRI scans, blood tests like CA-125, and genetic testing to help in diagnosis. In some cases, a biopsy may be needed to confirm the presence of cancer. Unfortunately, most women are diagnosed in later stages, which significantly impacts their prognosis.
Treatment typically involves surgery to remove the tumor and affected ovaries, followed by chemotherapy to target remaining cancer cells. For advanced or recurrent cases, targeted therapy and immunotherapy may be used. Newer treatments like immunotherapy show promise in improving survival rates.
Read more about Ovarian Cancer: Symptoms, Causes, Stages, Diagnosis and Treatment on OncoDaily.
Written by Mariam Khachatryan, MD


