This is an overveiw of multicenter, retrospective study, which evaluates the efficacy and safety of avelumab maintenance therapy in advanced urothelial carcinoma (aUC) patients following platinum-based chemotherapy (PBC), conducted across 13 Portuguese centers. The study demonstrates that avelumab significantly improves survival, with a median overall survival (OS) of 39.5 months, the longest reported to date. It also highlights the impact of infusion timing, suggesting that morning infusions may improve outcomes. The research further underscores the importance of treatment sequencing, with subsequent therapies like enfortumab vedotin (EV) showing superior progression-free survival (PFS) compared to chemotherapy. These findings support avelumab’s clinical benefit and call for further investigation into chrono-immunotherapy and optimized treatment strategies.
Title: Avelumab Maintenance Therapy in Advanced Urothelial Carcinoma: Implications of Timing and Treatment Sequencing
Authors: Lisa Gonçalves, Helena Guedes, Ana Raquel Fortuna, Tânia Lemos, João Gramaça, Natacha Mourão, Gonçalo Cunha, Rita Pichel, Pedro Simões, Joana Alves Luís, Rita Freitas, Inês Dunões ,Ana Sofia Spencer, Joana Marinho, Luís Costa
Published in Cancers, March 2025
Background
This multicenter study evaluates the real-world outcomes of avelumab maintenance therapy in patients with advanced urothelial carcinoma (aUC) following platinum-based chemotherapy (PBC). It also explores the impact of infusion timing on treatment efficacy.
Urothelial carcinoma (UC) is the most common type of bladder cancer, which remains difficult to treat, especially in advanced stages. It is the 9th most diagnosed cancer worldwide and a leading cause of cancer-related deaths. Traditionally, the standard first-line treatment for advanced UC has been platinum-based chemotherapy (PBC), with regimens such as cisplatin-gemcitabine (CG) and methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC). For patients ineligible for cisplatin, gemcitabine-carboplatin is an alternative. Recent genomic research suggests UC’s potential responsiveness to immune checkpoint inhibitors (ICIs) due to high tumor mutational burden and PD-L1 expression.
Study design and Methods
This was a multicentric, retrospective cohort study conducted across 13 Portuguese centers between September 2021 and September 2023. The study included patients with advanced urothelial carcinoma (aUC) who received maintenance immunotherapy with avelumab. Patients were followed until the date of death or the last medical record entry on 30 April 2024. The Data were retrieved from the patients’ electronic medical records, including demographic and clinical-pathological characteristics, infusion reactions, immune-related adverse events (irAEs), imaging responses, and the timing of avelumab administration.
Patient characteristics, treatment responses, progression-free survival (PFS), overall survival (OS), safety, and the impact of infusion timing were assessed.
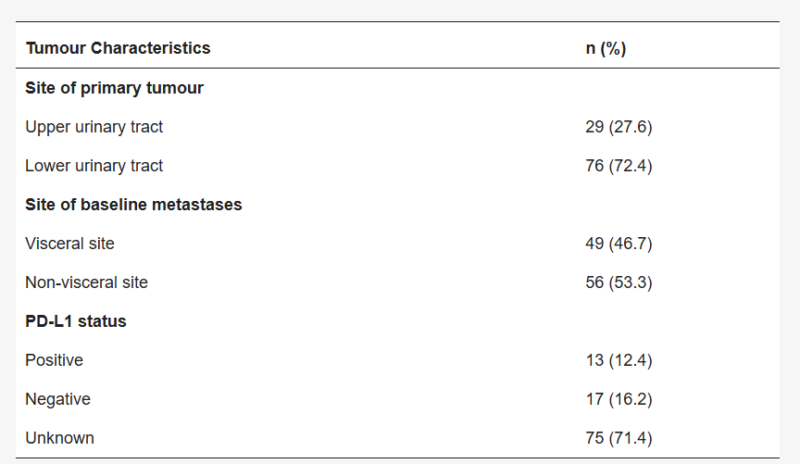
The cohort included 105 patients, predominantly male (78.1%), with a median age of 70 years and most of them had bladder tumors (72.4%) and stage IV disease at diagnosis (75.2%). Only in 28.6% of patients a PD-L1 testing was conducted from which 17 patients had a PD-L1 CPS ≥10. 53.3% of patients had no visceral metastases; common metastatic sites included lymph nodes (65.4%), lung (35.2%), liver (14.3%), and bone (14.3%).
The timing of avelumab infusions was dichotomized into morning (8 a.m.–2 p.m.) and afternoon (2 p.m.–8 p.m.) groups. Also Patients were categorized based on the proportion of treatments received in the afternoon. The morning group included patients who received less than 75% of infusions after 2 p.m., while the afternoon group included patients who received at least 75% of infusions after 2 p.m.
Results
The results of this retrospective study assessing avelumab in advanced urothelial carcinoma (aUC) patients demonstrate the drug’s clinical efficacy, safety, and suggest that the timing of infusion may influence patient outcomes. The study evaluated progression-free survival (PFS), overall survival (OS), and immune-related adverse events (irAEs), providing key insights into the treatment’s impact.
First-Line Treatment & Response:
- Most patients (54.3%) received cisplatin plus gemcitabine.
- The median interval between the last PBC cycle and avelumab initiation was 7 weeks.
- Response rates post-PBC: Pa4b rtial response (54.3%), complete response (8.6%), stable disease (32.3%).
- Median follow-up from avelumab initiation: 17.7 months.
Survival outcome
- Median PFS (mPFS): 9.8 months (95% CI, 4.9–14.7).
- Median OS (mOS): 39.5 months (95% CI, 13.2–65.7).
- Visceral metastases correlated with poorer survival (mPFS: 6.7 vs. 17.2 months, p = 0.034; mOS: 16.4 vs. 39.6 months, p = 0.017).
- Long-term disease control (>12 months) was observed in 32.4% of patients, significantly associated with PD-L1 CPS ≥10 (p = 0.031) and Grade 3 immune-related adverse events (irAEs) (p = 0.022).
Safety Data:
- irAEs occurred in 65.8% of patients, mostly Grade 1/2.
- Common irAEs: asthenia (n = 31), pruritus (n = 19), anorexia (n = 16), rash (n = 14), thyroid dysfunction (n = 14), nausea (n = 11).
- Infusion reactions occurred in 6.7% (1 case of Grade 4 respiratory acidosis and stridor).
- 8.6% discontinued avelumab due to irAEs.
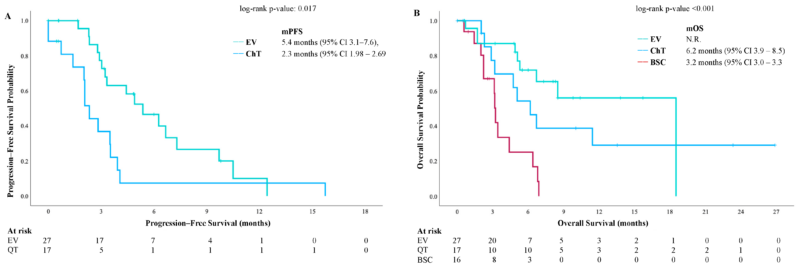
Subsequent Therapy Post-Avelumab:
- 67% of progressing patients received further treatment (ADC: 43%, ICI: 3.4%, chemotherapy: 20.7%).
- Enfortumab vedotin (EV) led to superior mPFS (5.4 months vs. 2.3 months for chemotherapy, p = 0.017) and improved OS (mOS not reached vs. 6.2 months for chemotherapy).
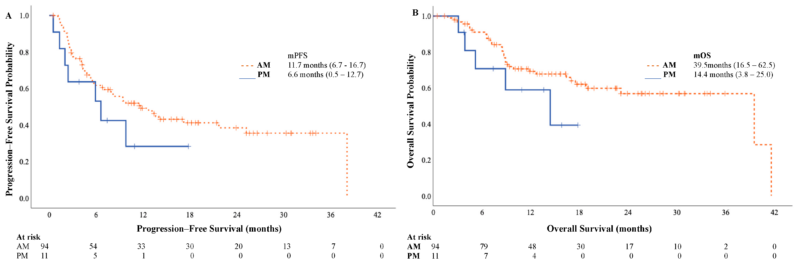
Chrono-Immunotherapy Analysis:
- Patients receiving <75% of avelumab infusions in the afternoon had improved mOS (39.5 vs. 14.4 months, p = 0.042) and a trend toward better mPFS (11.7 vs. 6.6 months, p = 0.055).
This study demonstrates that avelumab maintenance therapy significantly prolongs survival in real-world aUC patients, with an mOS of 39.5 months—the longest reported to date. The sequence of PBC, avelumab, and ADC therapy upon progression enhances patient outcomes. Additionally, findings suggest chrono-immunotherapy may influence treatment efficacy, warranting further prospective investigation.
Key Takeaways
Avelumab maintenance therapy significantly improves survival in real-world aUC patients, with a sequencing strategy of PBC → avelumab → ADC enhancing outcomes. Infusion timing may impact efficacy, highlighting the potential role of chrono-immunotherapy.
You Can Read the Full Article Here
Written by Sona Karamyan, MD