At the 2025 San Antonio Breast Cancer Symposium (SABCS), investigators presented the primary results of the phase III ASCENT-07 trial, evaluating sacituzumab govitecan (SG) versus treatment of physician’s choice chemotherapy (TPC) as first chemotherapy for patients with hormone receptor–positive, HER2-negative metastatic breast cancer following progression on endocrine therapy.
Background and Rationale
In the first-line metastatic setting, most patients with HR+/HER2− breast cancer receive endocrine therapy combined with a CDK4/6 inhibitor. Upon progression, additional endocrine-based strategies may be used; however, once disease becomes endocrine-refractory, treatment typically shifts to sequential single-agent chemotherapy.
Sacituzumab govitecan is a Trop-2–directed antibody–drug conjugate that has demonstrated statistically significant and clinically meaningful improvements in both progression-free survival and overall survival compared with chemotherapy in endocrine-refractory HR+/HER2− metastatic breast cancer following chemotherapy, as shown in the phase III TROPiCS-02 trial. ASCENT-07 was designed to evaluate whether SG could improve outcomes when used earlier, as the first chemotherapy after endocrine therapy.
Study Design
ASCENT-07 is a global, randomized, open-label phase III trial enrolling patients with locally advanced unresectable or metastatic HR+/HER2− breast cancer who had not received prior chemotherapy for advanced disease and were candidates for first chemotherapy.
- Patients were required to have measurable disease and to meet at least one of the following criteria:
Progression on ≥2 prior lines of endocrine therapy with or without a CDK4/6 inhibitor or targeted therapy. - Progression within 6 months of starting first-line endocrine therapy without a CDK4/6 inhibitor in the metastatic setting.
- Recurrence within 24 months of starting adjuvant endocrine therapy with a CDK4/6 inhibitor and no longer considered candidates for additional endocrine therapy.
A total of 690 patients were randomized 2:1 to receive:
- Sacituzumab govitecan 10 mg/kg IV on days 1 and 8 of a 21-day cycle (n=456), or
- Treatment of physician’s choice chemotherapy (capecitabine or taxane; n=234)
Randomization was stratified by duration of prior CDK4/6 inhibitor therapy, HER2 IHC status (IHC 0 vs HER2-low), and geographic region.The primary endpoint was progression-free survival (PFS) by blinded independent central review (BICR).Key secondary endpoints included overall survival (OS), objective response rate (ORR) by BICR, and quality of life. Additional secondary endpoints included investigator-assessed PFS and ORR, duration of response, and safety.
Statistical Considerations
Enrollment was planned for approximately 654 participants. A hierarchical testing procedure was used to control type I error, with PFS by BICR tested first at a two-sided 5% significance level. At the September 15, 2025 data cutoff, 419 PFS events and 187 OS events had occurred. Median follow-up was 15.4 months, and the study had 99% power to detect a PFS hazard ratio of 0.64.
Baseline characteristics were well balanced between treatment arms. Median age was approximately 57 years. HER2-low disease by immunohistochemistry was present in 58% of patients, with 42% classified as IHC 0. Primary endocrine resistance was reported in 31% of patients in the SG arm and 26% in the TPC arm. Approximately 90% of patients had visceral disease, and 70% had liver metastases. Nearly 91% had received a CDK4/6 inhibitor with endocrine therapy.
Primary Endpoint: PFS by BICR
At the primary analysis, ASCENT-07 did not meet its primary endpoint. Median PFS by BICR was 8.3 months in both treatment arms, with a stratified hazard ratio of 0.85 (P=0.130), which did not reach statistical significance.
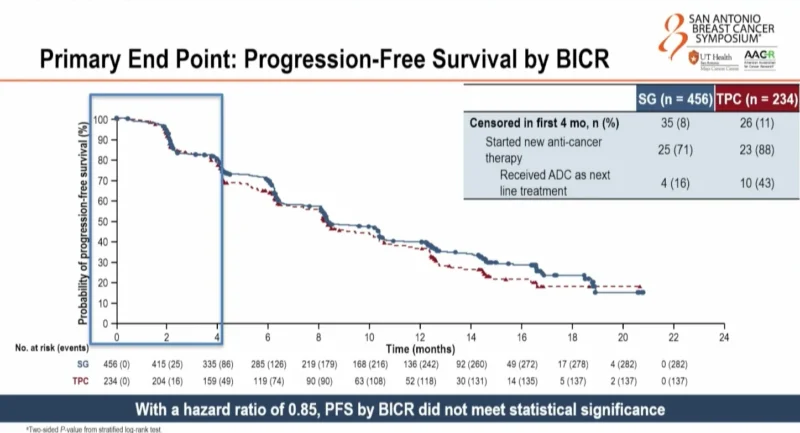
Early censoring within the first four months occurred in 8% of patients in the SG arm and 11% in the TPC arm, primarily due to initiation of new anticancer therapy. Among patients censored for starting new therapy, a higher proportion in the TPC arm received an antibody–drug conjugate as the next line of treatment.
Secondary and Exploratory Efficacy Endpoints
Investigator-assessed PFS, a key secondary endpoint, showed a numerical improvement with SG, with median PFS of 8.4 months versus 6.4 months with TPC (HR 0.78, nominal P=0.008). Six- and twelve-month PFS rates were numerically higher with SG.
PFS across prespecified subgroups was generally consistent with the overall population. In patients with HER2 IHC 0 disease, median PFS was 9.2 months with SG versus 8.1 months with TPC (HR 0.75), though confidence intervals crossed unity. Subgroup results were consistent regardless of prior CDK4/6 inhibitor exposure, choice of chemotherapy, or endocrine resistance status.
Overall Survival
Overall survival was included in the statistical hierarchy but is reported descriptively because the primary endpoint was not met. At 27% maturity, there was an early trend favoring SG, with a hazard ratio of 0.72 and a nominal P-value of 0.029. Median OS was not reached in either arm. Interpretation is limited by immature data and substantial use of subsequent therapies, including antibody–drug conjugates, in the TPC arm.
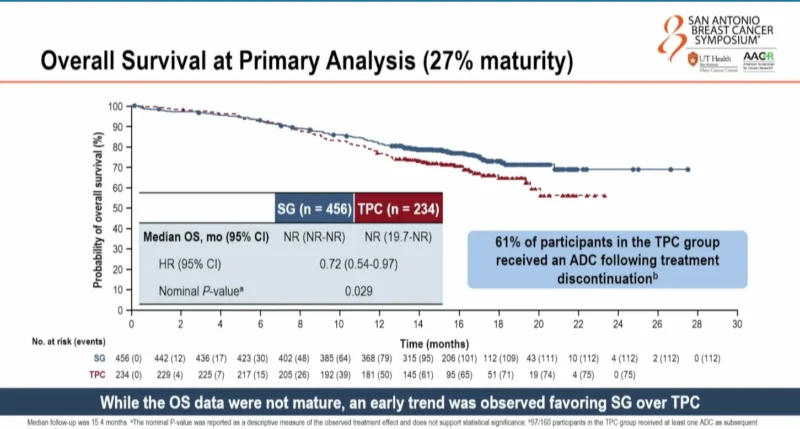
Following treatment discontinuation, 61% of patients in the TPC arm received at least one subsequent ADC compared with 32% in the SG arm. Trastuzumab deruxtecan was received by 41% of patients in the TPC arm and 29% in the SG arm. SG was used as a subsequent ADC in 18% of patients in the TPC arm.
Tumor Response
Objective response rates were similar between treatment arms (37% with SG vs 33% with TPC). Clinical benefit rate was numerically higher with SG (65% vs 53%). Responses were more durable with SG, with a median duration of response of 12.1 months compared with 9.3 months for TPC.
Safety
The safety profile of SG was consistent with its known toxicity profile. Median duration of treatment was 8.3 months with SG and 6.1 months with TPC. Grade ≥3 treatment-related adverse events occurred more frequently with SG (68% vs 37%); however, when neutropenia was excluded, rates were more comparable (36% vs 28%).
Treatment discontinuation due to adverse events was lower with SG (3% vs 7%). Treatment-emergent adverse events leading to death occurred in 2% of patients in both arms. The most common grade ≥3 adverse events in both groups were neutropenia, leukopenia, and anemia. Growth-factor prophylaxis was more frequently used in the SG arm.
Conclusions
ASCENT-07 did not meet its primary endpoint of PFS by blinded independent central review when evaluating sacituzumab govitecan versus physician’s choice chemotherapy as first chemotherapy in HR+/HER2− metastatic breast cancer. Investigator-assessed PFS showed a numerical improvement with SG, and an early, descriptive trend toward improved overall survival was observed at immature follow-up. Objective response rates were similar between arms, while duration of response favored SG. The safety profile of SG was manageable and consistent with prior studies, with lower treatment discontinuation rates compared with chemotherapy.
While SG did not demonstrate a statistically significant PFS benefit in this setting, it remains a standard of care after prior endocrine therapy and chemotherapy in HR+/HER2− metastatic breast cancer based on the TROPiCS-02 trial.
For more information click here.


