Antibody-drug conjugates (ADCs) are one of the most important advances in modern cancer treatment. For many patients, especially those whose cancer has returned or stopped responding to standard therapies, ADCs offer a new option that combines precision targeting with potent anti-cancer drugs. Understandably, patients and families often ask a simple but crucial question: What is the antibody-drug conjugate success rate, and what does success really mean in real life?
To answer this, it is essential to look beyond headlines and understand how ADCs work, how success is measured in oncology, and what current clinical data show across different cancer types.
What Are Antibody-Drug Conjugates?
Antibody-drug conjugates are targeted cancer therapies designed to deliver chemotherapy directly to cancer cells while limiting exposure to healthy tissues. Each ADC has three key components: a monoclonal antibody that recognizes a specific marker on cancer cells, a highly potent cytotoxic drug, and a linker that connects the two.
The antibody acts like a guided missile, binding to a protein that is overexpressed on tumor cells. Once the ADC is internalized, the linker releases the drug inside the cancer cell, leading to cell death. This targeted delivery is what distinguishes ADCs from traditional chemotherapy and underpins expectations about improved effectiveness and tolerability.
How Is the Antibody-Drug Conjugate Success Rate Measured?
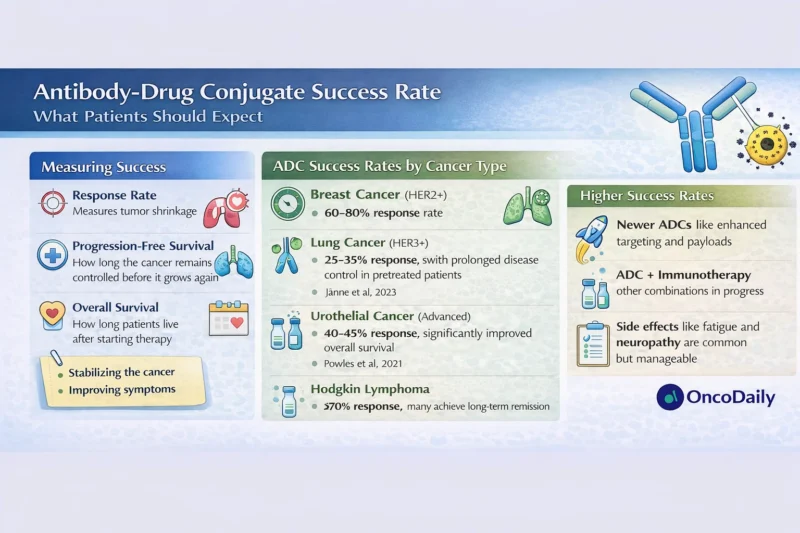
The antibody-drug conjugate success rate is not a single number and does not mean the same thing for every patient. In clinical trials and real-world practice, success is assessed using several well-established oncology outcomes.
Objective response rate refers to the percentage of patients whose tumors shrink by a predefined amount on imaging. Progression-free survival measures how long the cancer remains controlled before it grows again. Overall survival reflects how long patients live after starting treatment, regardless of cause of death. For patients, quality of life and symptom control are also critical dimensions of success, even when tumors do not dramatically shrink.
Importantly, a meaningful antibody-drug conjugate success rate may involve tumor stabilization, delayed progression, or improved symptoms rather than complete remission.
Antibody-Drug Conjugate Success Rate Across Major Cancers
Breast Cancer
Breast cancer is where ADCs have demonstrated some of their most striking results. In HER2-positive metastatic breast cancer, trastuzumab deruxtecan has shown objective response rates of approximately 60–80% in patients previously treated with multiple lines of therapy, with median progression-free survival exceeding 15 months in some studies (Modi et al., 2022). These outcomes represent a major improvement over older treatments.
Even in HER2-low breast cancer, a population previously considered unlikely to benefit from HER2-targeted therapy, ADCs have significantly improved progression-free and overall survival compared with chemotherapy (Modi et al., 2022). For many patients, this has redefined expectations of treatment benefit.
Lung Cancer
In non-small cell lung cancer, ADCs targeting HER3 and other surface proteins have shown response rates in the range of 25–35% in heavily pretreated patients (Jänne et al., 2023). While these numbers may appear modest compared with breast cancer, they are clinically meaningful in settings where few effective options exist.
Here, the antibody-drug conjugate success rate is often reflected more in prolonged disease control and symptom relief than in dramatic tumor shrinkage.
Urothelial (Bladder) Cancer
In metastatic urothelial cancer, enfortumab vedotin has become a standard therapy after chemotherapy and immunotherapy failure. Clinical trials have demonstrated response rates around 40–45%, with a significant improvement in overall survival compared with standard chemotherapy (Powles et al., 2021).
For patients with advanced bladder cancer, this represents one of the highest success rates seen in late-line treatment and has changed the treatment landscape.
Read About Antibody-Drug Conjugates on OncoDaily
Hematologic Malignancies
ADCs have also transformed outcomes in some blood cancers. Brentuximab vedotin, used in Hodgkin lymphoma, achieves response rates exceeding 70% in relapsed disease and has improved long-term survival when incorporated into earlier lines of therapy (Connors et al., 2018).
In this context, the antibody-drug conjugate success rate includes not only response but also the ability to achieve durable remissions and, in some cases, cure.
What Factors Influence the Antibody-Drug Conjugate Success Rate?
The success of ADC therapy depends on several patient- and tumor-specific factors. The presence and level of the target antigen on cancer cells is critical. Tumors with higher expression of the target tend to respond better, although newer ADCs show activity even at low expression levels.
Previous treatments also matter. ADCs are often used after chemotherapy or immunotherapy, and heavily pretreated cancers may be more resistant. At the same time, some ADCs remain effective precisely because their payloads are more potent than conventional chemotherapy.
Patient health, organ function, and ability to tolerate side effects also influence outcomes. A therapy may be highly active biologically but still challenging for patients with frailty or significant comorbidities.
Side Effects and Their Impact on Perceived Success
From a patient perspective, the antibody-drug conjugate success rate is closely linked to tolerability. While ADCs are more targeted than chemotherapy, they are not free of side effects.
Common toxicities include fatigue, nausea, low blood counts, and hair loss. Some ADCs have unique risks, such as peripheral neuropathy or lung inflammation, which require careful monitoring. Importantly, many patients find ADC side effects more manageable than traditional chemotherapy, particularly when dose adjustments and supportive care are used.
A treatment that controls cancer but severely limits daily functioning may not feel successful to a patient. This is why patient-reported outcomes are increasingly incorporated into ADC trials.
Real-World Expectations Versus Clinical Trial Results
Clinical trial data often represent the best-case scenario, with carefully selected patients and close monitoring. In real-world practice, antibody-drug conjugate success rates may be slightly lower, but they remain clinically meaningful.
Real-world studies of enfortumab vedotin and trastuzumab deruxtecan have confirmed that many patients experience tumor shrinkage or disease stabilization, even outside trial settings (Rosenberg et al., 2023). These findings help reassure patients that benefits seen in trials are not limited to academic centers.
Can Antibody-Drug Conjugates Cure Cancer?
For most solid tumors, ADCs are not considered curative when used in advanced disease. Instead, their primary role is to extend survival, control symptoms, and improve quality of life. In earlier-stage settings and certain blood cancers, ADCs may contribute to long-term remission and potential cure, especially when combined with other therapies.
Understanding this distinction is crucial when interpreting the antibody-drug conjugate success rate. Success often means living longer and better, not necessarily eliminating cancer completely.
The Future of Antibody-Drug Conjugate Success Rates
The field of ADC development is advancing rapidly. Newer linkers, more stable antibodies, and novel payloads are improving both efficacy and safety. Combination strategies with immunotherapy and targeted agents are also under investigation and may further enhance success rates.
As these innovations mature, antibody-drug conjugate success rates are expected to improve, particularly as therapies move into earlier lines of treatment where cancers are less resistant.
What Patients Should Take Away
The antibody-drug conjugate success rate varies widely depending on cancer type, disease stage, and individual patient factors. For many patients with advanced cancer, ADCs offer higher response rates and longer disease control than older therapies, with a side-effect profile that is often more tolerable.
Patients considering ADC therapy should discuss expected benefits, potential risks, and realistic goals with their oncology team. Understanding what “success” means in the context of personal values and quality of life is just as important as numerical response rates.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


