The Saudi Arabian Food and Drug Authority has approved Anktiva, an IL-15–based immunotherapy developed by Patrick Soon-Shiong and his biotechnology company ImmunityBio, for the treatment of bladder cancer and lung cancer, marking the first national regulatory authorization of this therapy beyond its current U.S. indication.
The decision represents a notable international expansion for Anktiva, which is presently approved by the Food and Drug Administration for a narrow indication—BCG-unresponsive, non–muscle-invasive bladder cancer (NMIBC) with carcinoma in situ. While U.S. regulators have declined to broaden its label to additional bladder cancer subtypes, Saudi authorities have taken a more expansive position, approving the therapy for two malignancies under their national regulatory framework.
Soon-Shiong framed the Saudi approval as both a scientific and policy inflection point.
“There’s now really significant proof, multiple trials, 10 years of work in which we’ve shown that this actually prolongs survival, whether it’s breast cancer, lung cancer and even pancreatic cancer, now glioblastoma. So this is a universal treatment,” Soon-Shiong told CUOMO on Wednesday.
He added:
“Hopefully, this could convince the current FDA that something invented in America should help Americans.”

Patrick Soon-Shiong
What Is Anktiva?
Anktiva is an IL-15 receptor agonist immunotherapy designed to activate and expand natural killer (NK) cells and memory CD8⁺ T cells. Unlike chemotherapy or gene therapy, Anktiva functions by amplifying endogenous immune effector pathways rather than directly targeting tumor cells.
According to ImmunityBio, Anktiva is:
“the first FDA-approved immunotherapy that activates what’s called a natural killer cell to target and kill non-muscle-invasive bladder cancer cells.”
Clinically, Anktiva is administered in combination with BCG (Bacillus Calmette-Guérin) for patients with NMIBC who have failed BCG alone. The drug is delivered intravesically via catheter, followed by maintenance therapy.
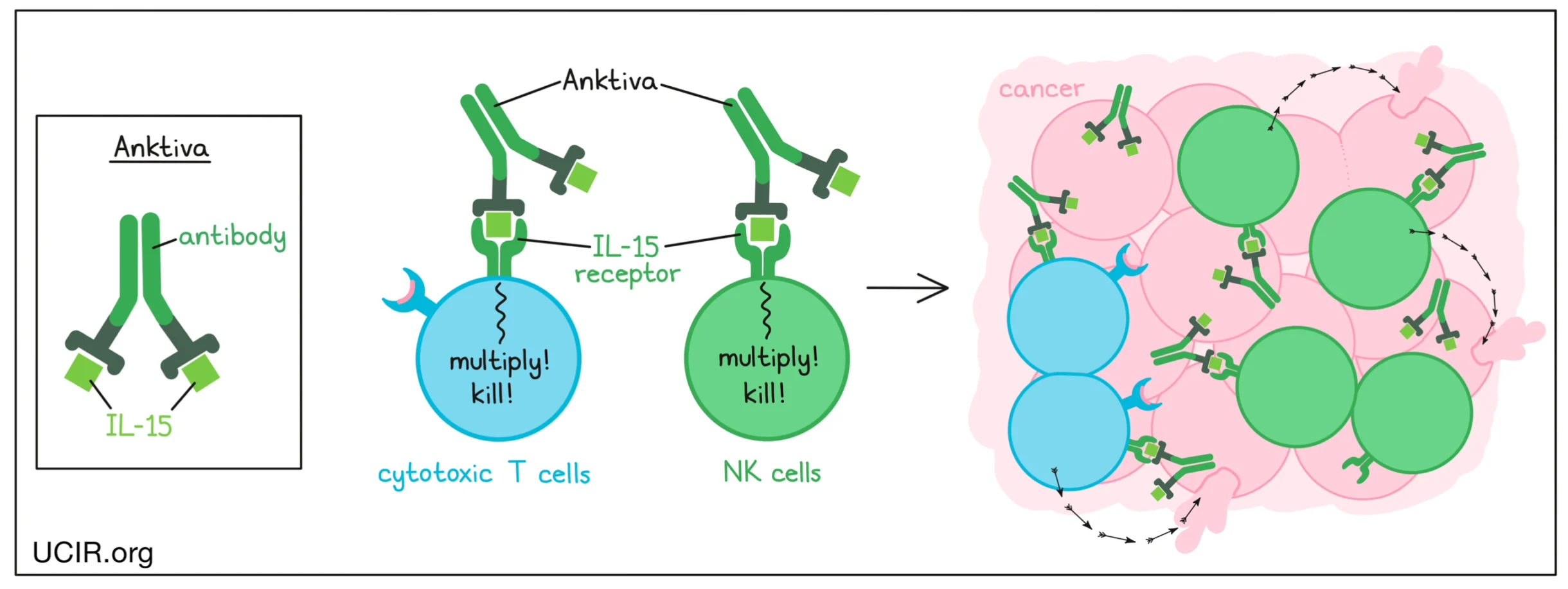
Soon-Shiong describes its mechanism succinctly:
“Anktiva is that IL-15. It is the switch that actually binds to the NK cell and activates and proliferates them.”
By enhancing NK-cell durability, proliferation, and cytotoxic capacity, Anktiva aims to re-engage immune surveillance in tumors that evade conventional immune checkpoint blockade.
Clinical Evidence Supporting FDA Approval in NMIBC
The FDA approval granted on April 22, 2024, was based on a single-arm clinical trial enrolling 77 patients with BCG-unresponsive stage 0 NMIBC. Patients received intravesical Anktiva plus BCG, with maintenance therapy extending up to 37 months.
Key efficacy findings included:
Complete response rate: 62%
Durability:
- 58% of responders remained disease-free at ≥12 months
- 40% remained disease-free at ≥24 months
These outcomes are clinically meaningful in a population where cystectomy is often the only curative option, but they were generated without a randomized control arm—an issue that continues to shape regulatory debate.
Regulatory Tension in the United States
Despite FDA approval in CIS-containing NMIBC, the agency declined to expand Anktiva’s indication to patients with papillary-only disease. ImmunityBio challenged the decision, arguing that the same dataset had already supported approval in a nearly identical population.
Former FDA principal deputy commissioner Rachel Sherman, MD, stated:
“… it is incomprehensible to me that the FDA refuses to file a supplemental BLA, stating the study is not sufficient to support a regulatory review, when it has already approved a product based on that very same study in essentially the same indication and population.”
The FDA has also raised concerns regarding promotional claims, issuing a warning letter citing unsupported survival and cystectomy-avoidance statistics on ImmunityBio’s healthcare provider website.
Saudi Approval: Broader Scope, Faster Translation
In contrast to the FDA’s incremental approach, Saudi regulators approved Anktiva for both bladder and lung cancer, signaling greater regulatory flexibility in the context of unmet clinical need.
Soon-Shiong emphasized the symbolic and strategic importance of this decision:
“We want this for our nation, and we’re not going to follow the FDA. We want to be first… this is now approved first for lung cancer and bladder cancer, and they offered that this will be approved for all other tumors as we proceed with this work now in Saudi Arabia.”
Saudi Arabia thus becomes the first country to authorize Anktiva beyond NMIBC, potentially positioning itself as a global testing ground for broader oncologic applications.
Safety Profile and Clinical Considerations
Reported adverse events with Anktiva largely reflect immune activation and localized bladder effects, including:
- Dysuria, urinary urgency, hematuria
- Fever, chills
- Musculoskeletal pain
- Transient laboratory abnormalities (creatinine, potassium)
While nearly 1,000 patients have reportedly received Anktiva across studies and expanded use, critics caution that broader claims—particularly across pancreatic cancer, glioblastoma, and lung cancer—require randomized, controlled validation.
Some in the oncology community have expressed concern that clinical enthusiasm may be outpacing confirmatory evidence.
Expert Perspective: Urgency Balanced With Evidence
Dr. Steven Finkelstein, national director of radiation oncology for U.S. Urology Partners, underscored the broader clinical context:
“There’s so many patients with cancers, afflicted with cancer, that we could do so much more for. It’s so important to figure out if it works, to get it to a place where everyone gets access.”

Dr. Steven Finkelstein
Soon-Shiong, who has self-funded the drug’s development, framed his motivation in humanitarian terms:
“The money spent to develop this drug over the last decade has been out of my pocket… The idea was to find a way to cure cancer. This has been a life dream, and we are very close now.”
Outlook
Anktiva represents a distinct immunologic strategy, centered on innate immune activation rather than checkpoint inhibition or cytotoxic therapy. The Saudi FDA’s approval introduces a new chapter in its development—one that may accelerate global clinical experience while intensifying scrutiny from regulators, clinicians, and scientists alike.
You Can Watch More on OncoDaily Youtube TV