Presented by Dr. Xiuning Le (Houston, USA) during Session LBA75 at ESMO 2025, results from the Phase I/II SOHO-01 study highlighted sevabertinib (BAY 2927088) — a potent, reversible, oral HER2 tyrosine kinase inhibitor (TKI) — as a promising targeted therapy for patients with advanced HER2-mutant non-small cell lung cancer (NSCLC). The drug, which has received FDA Breakthrough Therapy Designation and Priority Review, demonstrated durable responses and a favorable safety profile in both pretreated and treatment-naïve patients.

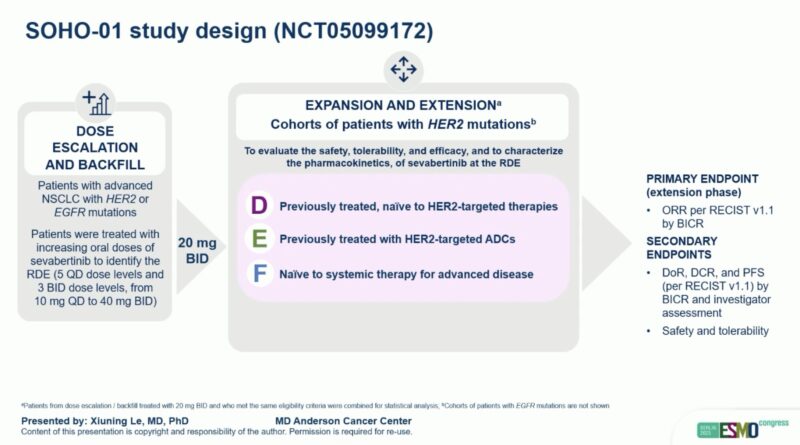
Study Design
A total of 209 patients with HER2-mutant NSCLC received sevabertinib 20 mg twice daily across three cohorts:
- Cohort D: Previously treated with systemic therapy but naïve to HER2 exon 20 insertion–targeted agents.
- Cohort E: Previously treated with HER2-directed antibody–drug conjugates (ADCs).
- Cohort F: Systemic-therapy–naïve for advanced disease.
The primary endpoint was objective response rate (ORR) per RECIST v1.1 by blinded independent central review (BICR). Secondary endpoints included duration of response (DoR) and progression-free survival (PFS).
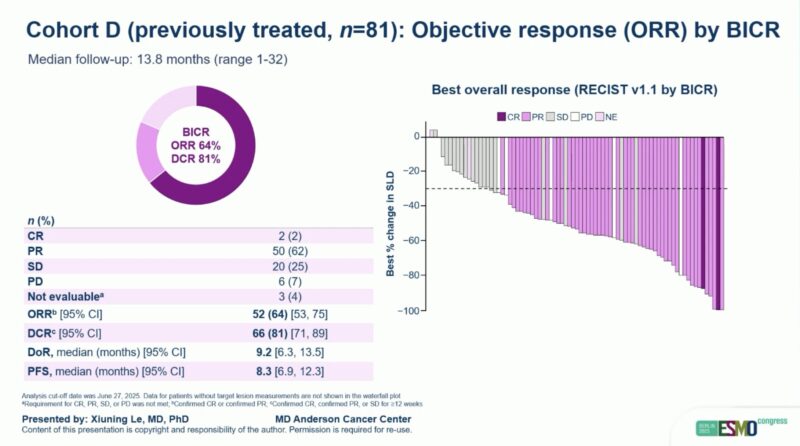
Efficacy Results
At the data cut-off (June 27, 2025), sevabertinib achieved notable responses across all cohorts:
- Cohort D: ORR 64% (95% CI 53–75); median DoR 9.2 months (95% CI 6.3–13.5); median PFS 8.3 months (95% CI 6.9–12.3).
- Cohort E: ORR 38% (95% CI 25–52); median DoR 8.5 months (95% CI 5.6–16.4); median PFS 5.5 months (95% CI 4.3–8.3).
- Cohort F: ORR 71% (95% CI 59–81); median DoR 11.0 months (95% CI 8.1–not evaluable); median PFS not yet reached.
Patients with baseline brain metastases in Cohort D achieved similar ORR (61%) compared to those without (65%). In patients harboring the HER2 Y772_A775dupYVMA mutation, outcomes were particularly favorable (ORR 78% vs 57%; median PFS 12.2 vs 7.0 months).
Safety Profile
Grade ≥ 3 treatment-related adverse events (TRAEs) occurred in 31% of patients. Diarrhea was the most frequent TRAEs, predominantly grade 1/2 (grade 3 in 14%). Treatment discontinuations due to TRAEs were rare (3%), and no cases of interstitial lung disease or pneumonitis were reported.
Conclusion
The SOHO-01 trial, presented at ESMO 2025, demonstrated that sevabertinib produces rapid, durable, and clinically meaningful responses with manageable toxicity in patients with advanced HER2-mutant NSCLC. These results support sevabertinib as a potential new oral targeted therapy that could redefine the treatment landscape for this molecular subset.
Trial registration: NCT05099172
Sponsor: Bayer AG
Funding and Editorial Support: Bayer AG and Caudex, IPG Health Medical Communications
You Can Also Read INTEGRATE IIb Trial at ESMO 2025: Regorafenib Plus Nivolumab vs Chemotherapy in Advanced Gastric and GEJ Cancer by OncoDaily
You Can Read Full Abstract Here
