At the ESMO Congress 2025 in Berlin, Dr. Silvia Marsoni (IFOM ETS – AIRC Institute of Molecular Oncology, Milan, Italy) presented the final results of the PEGASUS trial (NCT04259944), a multicenter, investigator-initiated study exploring a circulating tumor DNA (ctDNA)-guided strategy to personalize adjuvant treatment after surgery for stage III and high-risk stage II colon cancer.
At OncoDaily GI, we spotlight innovations reshaping GI oncology — from perioperative immunotherapy to molecularly guided minimal residual disease (MRD) surveillance that could redefine postoperative care.
Background
Despite curative resection and standard adjuvant chemotherapy, up to one-third of patients with stage III colon cancer relapse within three years.
Recent evidence shows that ctDNA detection after surgery is a strong, independent prognostic marker for recurrence, identifying patients with molecular residual disease well before radiologic relapse.
The PEGASUS trial was designed to test whether liquid-biopsy results could safely guide escalation or de-escalationof adjuvant therapy — reducing overtreatment for ctDNA-negative patients while intensifying therapy for those at highest molecular risk.
PEGASUS Trial Design
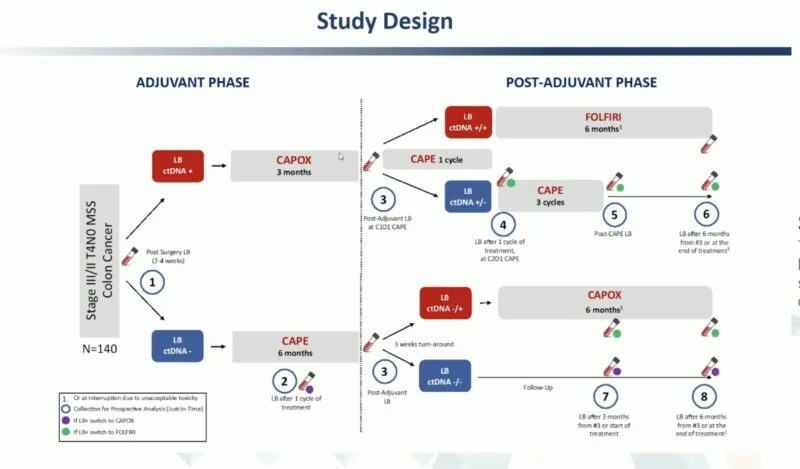
This prospective, phase II study enrolled 135 patients with stage III or T4N0 stage II colon cancer at 11 centers in Italy and Spain.
All patients underwent ctDNA testing using the Guardant Reveal vL1.2 assay shortly after surgery.
- ctDNA-positive (LB+) patients received 3 months of CAPOX.
- ctDNA-negative (LB–) patients received 6 months of capecitabine (CAPE).
After one cycle of CAPE, a second liquid biopsy was performed; if positive, therapy was escalated to CAPOX.
At completion of adjuvant therapy, ctDNA testing was repeated:
- For CAPOX-treated patients:
- LB+ → switch to FOLFIRI
- LB– → de-escalate to CAPE
- For CAPE-treated patients:
- LB+ → switch to CAPOX
- LB– → standard follow-up
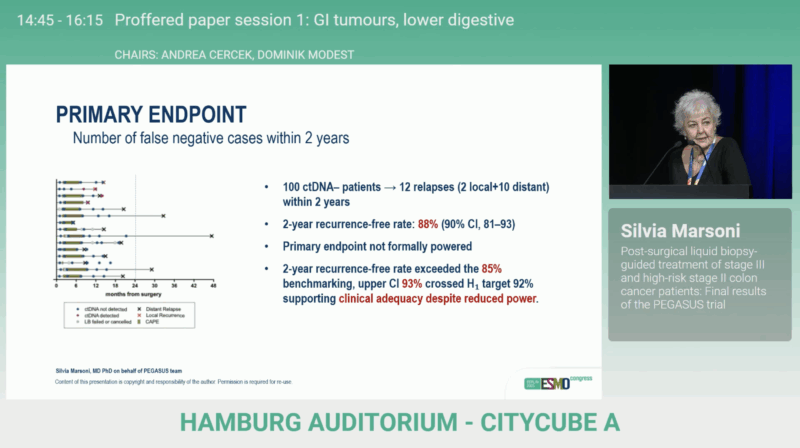
The primary endpoint was feasibility, defined as the 2-year disease-free rate among patients with two consecutive postoperative LB– results.
A ≤ 14 relapses threshold among 134 LB– patients was required to confirm the pre-specified alternative hypothesis (H₁ = 92 % vs H₀ = 85 %).
A historical comparison with the TOSCA trial was planned as a secondary analysis.
Previous Results from ESMO 2023
In this phase II study, presented at ESMO 2023, 135 patients across Italy and Spain underwent ctDNA testing after surgery using Guardant Reveal. ctDNA-positive patients received CAPOX, while ctDNA-negative patients received CAPE, with repeat testing to adjust therapy and reduce false negatives. At the end of treatment, ctDNA results guided escalation (to FOLFIRI) or de-escalation.
At a median follow-up of 20.8 months, 26 % of patients were ctDNA-positive, showing a markedly higher relapse risk (HR 4.37; p = 0.0003).
Switching ctDNA-positive patients to FOLFIRI converted roughly 50 % to ctDNA-negative, with many remaining relapse-free.
Only nine ctDNA-negative patients relapsed, supporting ctDNA as a powerful early predictor of outcome and a tool for refining adjuvant therapy.
Results from ESMO 2025
From July 2020 to July 2022, 135 patients (100 LB–, 35 LB+) were enrolled across 11 centers in Italy and Spain.
With a median follow-up of approximately 42 months (41 months for LB– and 44 months for LB+ patients), 12 relapses occurred among LB– patients, yielding a 2-year DFS rate of 88% (90% CI 81–93).
Although the pre-defined feasibility threshold was narrowly missed, the observed performance exceeded the 85% benchmark, supporting the practicality of ctDNA-guided management.
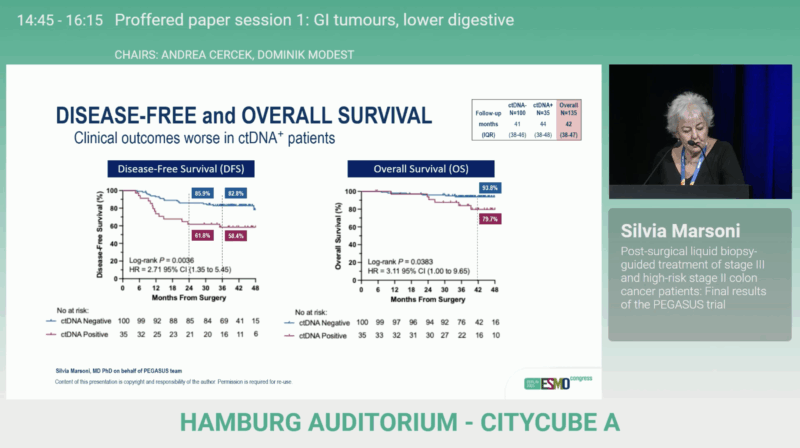
Among LB+ patients, 13 relapses (37%) were observed, with 2-year DFS rates of 85.9% in LB– vs 61.8% in LB+ (HR 2.71; 95% CI 1.35–5.45; p=0.0036), confirming the strong prognostic value of ctDNA positivity. Overall survival also favored LB– patients (93.8% vs 79.7%; HR 3.11; 95% CI 1.00–9.65; p=0.0383), consistent with the disease-free survival advantage.
Conclusions
The PEGASUS trial demonstrates the operational feasibility and clinical promise of liquid biopsy-guided adjuvant therapy in resected stage III and high-risk stage II colon cancer.
By tailoring treatment duration and intensity to molecular risk, this approach may reduce unnecessary toxicity and optimize postoperative surveillance.
A historical comparative analysis versus the TOSCA cohort is ongoing to contextualize survival outcomes.
The study was sponsored by IFOM ETS – AIRC Institute of Molecular Oncology and funded by Guardant Health.

You can read the full abstract here.
