Radiotherapy (RT) has long been a backbone of cancer therapy, appreciated for its capacity to deliver lethal DNA damage to tumor cells in a localized fashion. Yet, the advent of immune checkpoint inhibitors (ICIs) has reshaped our understanding of cancer treatment—transforming it into a systemic, immune‑mediated process.
Increasingly, evidence suggests that RT is not merely a cytotoxic tool: it can act as an immunologic adjuvant, promoting antigen release, dendritic cell activation, T cell priming, and even systemic “abscopal” tumor regression.This evolving paradigm has spurred interest in combining RT with immunotherapy (IO). By turning immunologically “cold” tumors into “hot,” inflamed ones, these combinations may enhance response rates across multiple cancer types.
In this article, I review the biologic rationale, timing strategies, clinical trial evidence with real-world outcomes, safety considerations, biomarker insights, and future directions of delivering immunotherapy during radiotherapy in a tumor-agnostic, evidence-based framework.
What Is the Abscopal Effect?
In modern oncology, the abscopal effect describes a rare but increasingly observed phenomenon where localized radiotherapy (RT) leads to the shrinkage of tumors at distant, non-irradiated sites. Once considered a biological curiosity, this effect has gained scientific credibility in the era of immunotherapy, particularly with the use of checkpoint inhibitors.
Mechanistically, RT induces immunogenic cell death, releasing tumor-associated antigens and pro-inflammatory signals that prime the immune system. When combined with immune checkpoint blockade, this localized immune activation can escalate into a systemic T cell–mediated anti-tumor response, reaching beyond the irradiated field.
Clinically, abscopal responses are now reported more frequently in studies combining RT with PD-1/PD-L1 inhibitors, though they remain unpredictable. This effect underscores a major shift in perspective: radiotherapy is not merely a local treatment but can act as an immune-stimulating adjuvant, unlocking systemic therapeutic potential when paired with immunotherapy.
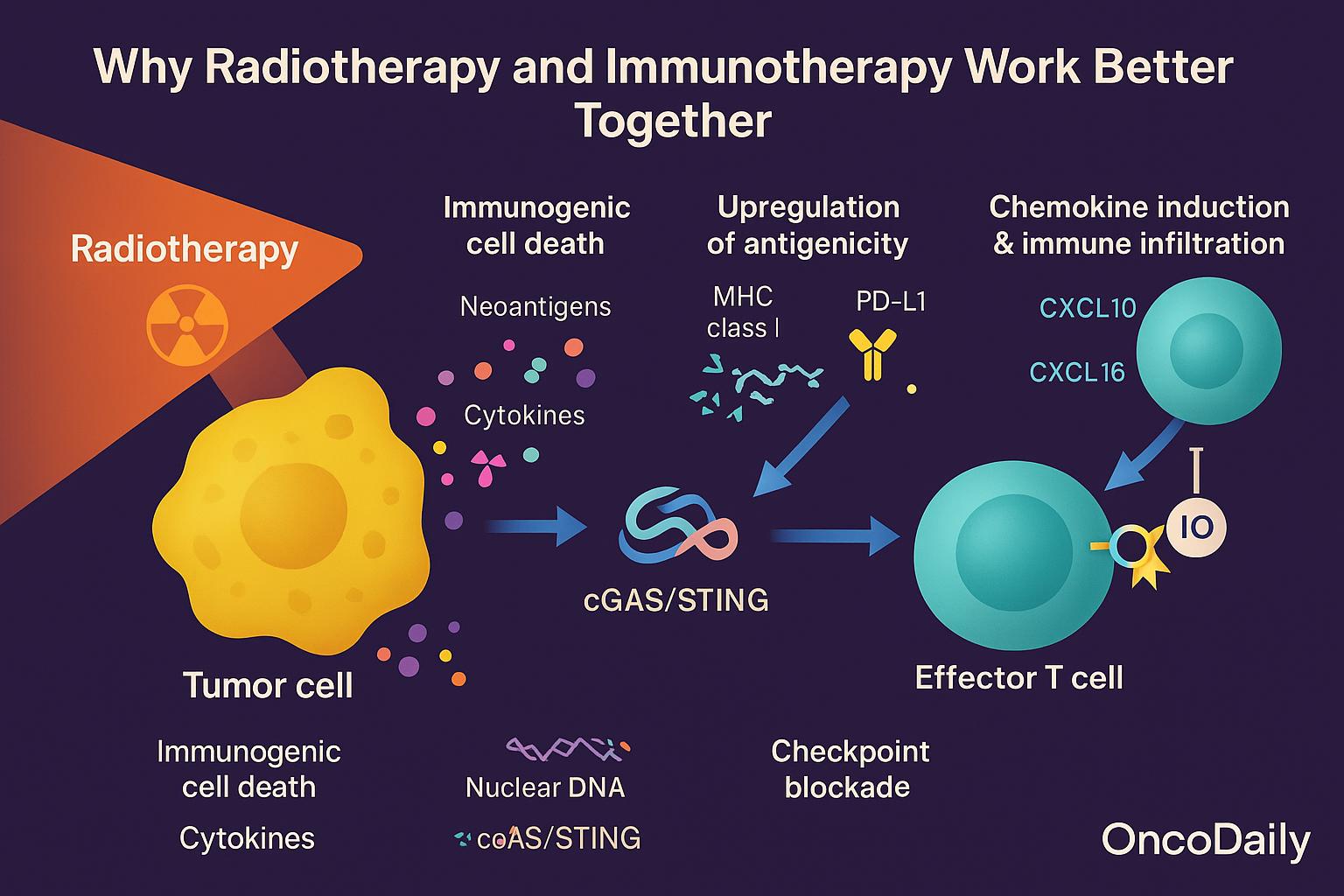
Why Radiotherapy and Immunotherapy Work Better Together
Why RT and IO Work Better Together: Mechanistic Rationale
The synergy between radiotherapy and immunotherapy rests on complementary, immune‑modulating effects:
- Immunogenic cell death (ICD): RT induces a form of apoptosis that is especially stimulatory to the immune system. As malignant cells die, they release danger signals (damage-associated molecular patterns, or DAMPs), neoantigens, and cytokines—activating antigen-presenting cells.
- cGAS/STING activation: RT can fragment nuclear DNA, enabling cytosolic sensing via the cGAS–STING pathway. This triggers type I interferon responses, dendritic cell activation, and cross-priming of T cells.
- Upregulation of antigenicity: RT also increases MHC class I expression and PD-L1 levels on tumor cells, thereby enhancing tumor visibility to cytotoxic T cells—even while exposing immune checkpoints to therapeutic targeting.
- Chemokine induction and immune infiltration: RT stimulates chemokines such as CXCL10 and CXCL16, which attract effector T cells into the tumor microenvironment, helping convert “immune-excluded” tumors into “inflamed” ones.
- Checkpoint blockade unleashes effectors: Once RT has primed and recruited T cells, IO (e.g., anti–PD-1, anti–CTLA-4) removes the inhibitory brakes, sustaining and amplifying antitumor immunity.
This phenomenon is often termed “immunogenic modulation”—the idea that RT not only kills tumor cells but reprograms them to be more susceptible to immune attack. By synchronizing priming (RT) and effector activation (IO), the combination approach can overcome multiple forms of immune resistance.
Timing Strategies for Combining Immunotherapy and Radiotherapy
When integrating immunotherapy (IO) with radiotherapy (RT), three primary timing strategies have emerged: concurrent, sequential, and sandwich approaches.
Concurrent administration refers to giving IO at the same time as RT. This strategy is designed to maximize synergy by exposing the immune system to tumor neoantigens released by RT while simultaneously blocking inhibitory checkpoints. However, it may also carry a higher risk of overlapping toxicities, such as pneumonitis or dermatitis, especially when combining immune checkpoint inhibitors with thoracic or high-dose RT.
Sequential administration involves delivering IO after the completion of RT. This approach may allow RT to debulk the tumor, modulate the microenvironment, and prime the immune system first, before introducing IO to sustain and amplify the immune response. The PACIFIC trial exemplifies this strategy—showing improved survival with durvalumab following chemoradiotherapy in unresectable stage III NSCLC.
Sandwich approaches combine IO before, during, and after RT in varying sequences. These regimens aim to “precondition” the immune system, enhance immune activation during RT, and sustain effector activity after RT. Early-phase studies are exploring these multi-phase regimens in solid tumors such as lung and head-and-neck cancers.
The key takeaway is that there is no one-size-fits-all strategy. The optimal timing depends on tumor type, IO mechanism of action, RT dose and fractionation schedule, and the kinetics of immune activation and recovery. Future trials should continue to dissect these variables to refine personalized timing strategies for combined IO and RT.
Clinical Trial Evidence for IO-RT Combinations Across Tumor Types
The clinical promise of combining immunotherapy (IO) with radiotherapy (RT) has been tested across tumor types—with mixed but illuminating results. Below we review key clinical trials and their outcomes.
Non–Small Cell Lung Cancer (NSCLC)
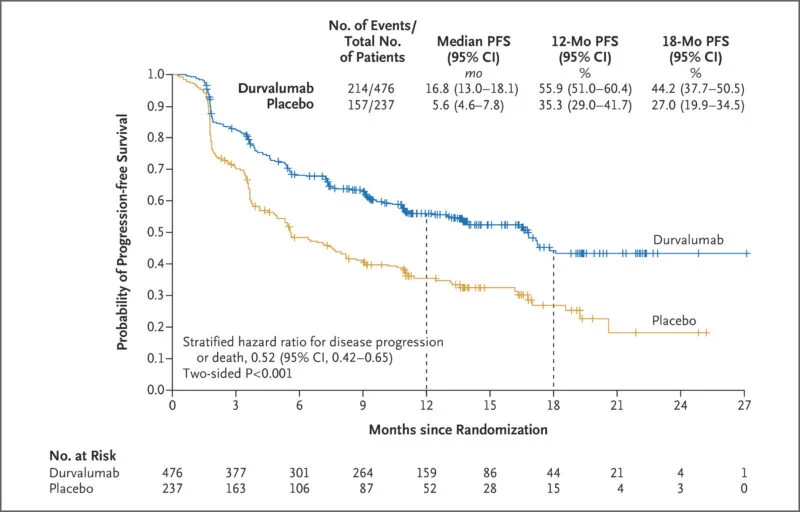
In stage III unresectable NSCLC, the PACIFIC trial established sequential IO after chemoradiation as a new standard of care. Patients with no disease progression after chemoradiotherapy were randomized to durvalumab vs placebo. The 12‑month progression-free survival (PFS) rates were 55.9% vs 35.3%, respectively (HR not specified here) New England Journal of Medicine. At 24 months, overall survival (OS) was 66.3% vs 55.6% in favor of durvalumab New England Journal of Medicine. With extended follow-up, ~49.6% of durvalumab-treated patients remained alive at 4 years compared to 36.3% in the placebo arm; PFS at 4 years was 35.3% vs 19.5% PubMed. Even at 5 years, 42.9% of durvalumab patients remained alive ACS Publications+1.
These results demonstrate a durable survival benefit with consolidation durvalumab, particularly when given after definitive RT.
There are also trials now exploring concurrent IO + RT in NSCLC—e.g., KEYNOTE-799, which administers pembrolizumab during chemoradiation. Early safety and efficacy signals are promising, though mature survival data are pending.
Head and Neck Squamous Cell Carcinoma (HNSCC)
The JAVELIN Head and Neck 100 trial investigated avelumab (anti–PD-L1) when combined concurrently with chemoradiotherapy (CRT) followed by maintenance. Unfortunately, the trial did not meet its primary endpoint of progression-free survival and showed no improvement in overall survival over CRT plus placebo ResearchGate+3PMC+3PubMed+3. Interim analyses indicated that adding the checkpoint inhibitor may have undermined the effect of CRT rather than synergizing with it ASCO Post.
Because this trial was negative, the HNSCC field has largely reverted to biomarker-driven approaches and further exploration of optimal timing and patient selection.
Glioblastoma (GBM)
In glioblastoma, trials combining IO and RT thus far have not improved outcomes. In CheckMate‑498 (for newly diagnosed MGMT-unmethylated GBM), nivolumab plus radiation failed to meet its primary endpoint of superior overall survival compared to standard temozolomide + RT Bristol Myers Squibb. Similarly, CheckMate‑548, testing nivolumab added to RT and temozolomide in MGMT-methylated patients, also did not demonstrate a survival benefit in published reports.
The lackluster outcomes are likely driven by the deeply immunosuppressive brain microenvironment, blood-brain barrier constraints, and frequent use of steroids that blunt immune responses.
Rectal and Esophageal Cancer
Although fewer trials are available in GI cancers, CheckMate-577 offers strong evidence in the esophageal / GEJ space. In patients who had residual disease after neoadjuvant chemoradiation and surgery, adjuvant nivolumab significantly improved disease-free survival: median DFS was 21.8 months vs 10.8 months with placebo (HR 0.76, 95% CI 0.63–0.91) OncLive+2Oncodaily+2. For overall survival, the median was 16.4 months longer in the nivolumab arm, though this difference did not reach formal statistical significance (HR 0.85, p = 0.1064) OncLive+2Oncodaily+2. However, long-term follow-up indicates a sustained DFS benefit and favorable 5-year OS trends, with manageable safety profiles Targeted Oncology+1.
In rectal cancer, ongoing trials are investigating checkpoint inhibitors integrated into chemoradiation regimens, particularly for MSI-H tumors. Data are still emerging, especially on pathological complete response rates and long-term outcomes.
Safety and Toxicity Considerations
While the combination of immunotherapy (IO) and radiotherapy (RT) holds significant therapeutic promise, it also raises critical concerns regarding toxicity profiles. Overlapping adverse effects between both modalities can lead to enhanced risk of immune-related adverse events (irAEs), particularly when delivered concurrently.
A prominent example is pneumonitis, which is observed with both thoracic RT and immune checkpoint inhibitors (ICIs) targeting PD-1 or PD-L1. In NSCLC patients, especially those receiving consolidation durvalumab after chemoradiation (as in the PACIFIC trial), the incidence of pneumonitis ranged from 5% to 20%, with most cases being manageable with corticosteroids. However, higher-grade events, while rare, necessitate prompt recognition and intervention.
Similarly, mucositis is a common sequela of head and neck RT, and when compounded by IO-induced inflammation, symptom severity may increase. In GI tumors, colitis represents a key toxicity concern, particularly with anti–CTLA-4–based regimens or in patients undergoing pelvic RT. These overlapping inflammatory toxicities demand close surveillance and early immunosuppressive intervention when necessary.
RT can also enhance antigen spread and immune activation, potentially magnifying systemic inflammation. This biologic synergy may paradoxically elevate the risk of irAEs beyond what is seen with IO alone. Immune flare, a phenomenon of exaggerated inflammation following local therapy, has been reported anecdotally and requires further study.
Importantly, ongoing clinical trials have generally demonstrated that toxicity from IO-RT combinations is manageable, especially with appropriate steroid use and supportive care. Nevertheless, timing and dosing are paramount—concurrent regimens may amplify toxicity, while sequential approaches may reduce peak inflammatory overlap. Patient selectionbased on tumor location, comorbidities, and immune baseline is essential to mitigate risks.

You Can Read About Side Effects of Radiotherapy: Risks and What to Know
Biological and Clinical Predictors of Response
As immunoradiotherapy strategies expand across tumor types, identifying predictive biomarkers becomes critical to refining patient selection, optimizing treatment timing, and maximizing clinical benefit. The biological interplay between radiotherapy (RT) and immunotherapy (IO) is deeply influenced by both tumor-intrinsic features and host immune contexture.
Tumor and Immune Biomarkers
Multiple biomarkers have emerged as potential predictors of response. PD-L1 expression remains one of the most clinically validated indicators, especially for checkpoint inhibitor therapies; however, its predictive value in the context of IO-RT combinations is more variable. Tumor mutational burden (TMB) and interferon-gamma (IFN-γ) gene signatures have also been linked to enhanced immune responsiveness and may correlate with benefit from RT-induced antigen release and T cell priming.
The gut microbiome is increasingly recognized as a modulator of immunotherapy efficacy, and emerging data suggest it may also shape responses to RT through systemic immune regulation. Similarly, circulating lymphocyte counts—both absolute and dynamic—may reflect immune competence and predict outcomes in IO-RT trials, particularly in lymphodepleting settings.
RT-Induced Immunologic Modulation
RT itself modifies the tumor microenvironment in ways that can either potentiate or hinder IO efficacy. It promotes infiltration by tumor-infiltrating lymphocytes (TILs) and enhances MHC class I expression, improving antigen presentation. These changes not only reflect RT’s capacity for in situ vaccination, but also provide measurable immunologic markers of response.
Moreover, RT fractionation and dose significantly influence immunogenic outcomes. Hypofractionated schedules (e.g., 8 Gy × 3 fractions) have been shown to induce greater immunogenic cell death, dendritic cell activation, and T cell priming compared to conventional low-dose daily RT. This has direct implications for clinical protocol design and may partially explain heterogeneous results across IO-RT trials.
Towards Biomarker-Guided Therapies
Recent and ongoing clinical trials increasingly incorporate biomarker-driven stratification, including PD-L1, TMB, and immune gene signatures, to guide treatment allocation and timing of IO in relation to RT. For instance, PD-L1 status informed patient selection in KEYNOTE-799, while translational substudies in rectal and esophageal cancer trials explore TIL density and IFN signatures as correlates of pathological response.
You Can Watch More on OncoDaily Youtube TV
