
FDA Approves Nivolumab and Ipilimumab for First-Line Treatment of Unresectable Hepatocellular Carcinoma Based on CheckMate-9DW Trial
April 11, 2025 — The U.S. Food and Drug Administration (FDA) has approved the combination of nivolumab (Opdivo) and ipilimumab (Yervoy) for the first-line treatment of adult patients with unresectable or metastatic hepatocellular carcinoma (HCC). This decision marks a significant expansion of the use of dual immune checkpoint blockade in liver cancer.
The FDA had previously granted accelerated approval for this combination in 2020 for patients with HCC who had been previously treated with sorafenib, based on durable responses in a multicenter study.
What is the rationale for FDA approval of Nivolumab and Ipilimumab in HCC?
The approval is based on the pivotal CheckMate-9DW (NCT04039607) trial, which demonstrated superior survival and response outcomes compared to the current standard therapies. CheckMate-9DW was a randomized (1:1), open-label, phase III trial involving 668 adults with histologically confirmed unresectable or metastatic HCC. Eligible patients had:
- Child-Pugh Class A liver function
- ECOG performance status 0 or 1
- No prior systemic therapy for advanced disease
Patients were randomized to receive
- Nivolumab 1 mg/kg IV + ipilimumab 3 mg/kg IV every 3 weeks for 4 doses, followed by
- Nivolumab 480 mg IV every 4 weeks as maintenance
or - Investigator’s choice of Lenvatinib (8 or 12 mg daily) or Sorafenib (400 mg twice daily)
Efficacy Outcomes
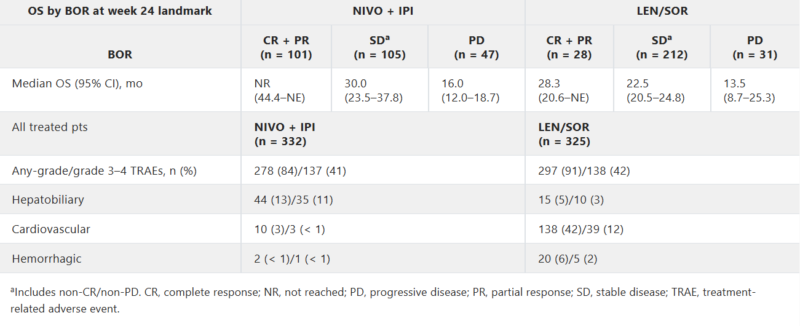
Primary Endpoint: Overall Survival (OS)
- Median OS with nivolumab/ipilimumab: 23.7 months (95% CI: 18.8–29.4)
- Median OS with sorafenib/lenvatinib: 20.6 months (95% CI: 17.5–22.5)
- Hazard Ratio (HR): 0.79 (95% CI: 0.65–0.96), P < 0.018
Secondary Endpoint: Objective Response Rate (ORR)
- ORR with nivolumab/ipilimumab: 36.1% (95% CI: 31.0–41.5)
- ORR with sorafenib/lenvatinib: 13.2% (95% CI: 9.8–17.3), P < 0.0001
Responses were assessed per RECIST v1.1 by blinded independent central review.
The 36-month OS rate was 38% in the immunotherapy arm versus 24% in the TKI arm, reflecting the durable benefit of checkpoint blockade. Notably, the median duration of response exceeded 30 months in the combination arm, more than doubling that observed with TKIs (12.9 months).
Safety and Dosing
The combination of nivolumab and ipilimumab was generally well-tolerated in patients with hepatocellular carcinoma. The most frequently reported side effects included rash, itching (pruritus), fatigue, and diarrhea—adverse events consistent with immune checkpoint blockade and manageable in most cases with supportive care and treatment modifications when necessary.
For patients with HCC, the recommended dosing schedule begins with nivolumab at 1 mg/kg combined with ipilimumab at 3 mg/kg, administered intravenously every three weeks for a total of four doses. This is followed by maintenance therapy with single-agent nivolumab, given either as 240 mg IV every two weeks or 480 mg IV every four weeks, depending on clinical preference and patient tolerance.
The CheckMate-9DW trial demonstrated durable survival, high response rates, and manageable toxicity, supporting its adoption as a key therapeutic strategy in advanced liver cancer. As oncologists navigate an increasingly complex treatment landscape, this regimen adds critical flexibility for personalizing care based on patient profile and treatment goals.
Read the full article in Journal of Clinical Oncology
Learn more about Opdivo (Nivolumab): Uses in Cancer, Side Effects, Dosages, Expectations On OncoDaily.
Accelerated Approval in the Second-Line Setting: CheckMate-040
On March 10, 2020, the FDA granted accelerated approval to the combination of nivolumab and ipilimumab for patients with HCC previously treated with sorafenib. This decision was based on results from Cohort 4 of the CheckMate-040 trial (NCT01658878), a multicenter, open-label trial that enrolled patients who had progressed on or were intolerant to sorafenib.
Cohort 4 Efficacy Results
- 49 patients received nivolumab 1 mg/kg + ipilimumab 3 mg/kg every 3 weeks (4 doses), followed by nivolumab 240 mg IV every 2 weeks
- Overall response rate (ORR) was 33% (95% CI: 20–48), with 4 complete responses and 12 partial responses
- 31% of responses lasted ≥24 months, and response duration ranged from 4.6 to 30.5+ months
Common Adverse Events
- Fatigue, diarrhea, rash, pruritus, nausea
- Musculoskeletal pain, pyrexia, cough, decreased appetite
- Vomiting, abdominal pain, dyspnea, URTI, arthralgia, headache
- Hypothyroidism, weight loss, dizziness
The dosing regimen used in this indication mirrors the approach established in the first-line setting. Patients initially receive a combination of nivolumab at 1 mg/kg and ipilimumab at 3 mg/kg, administered intravenously every three weeks for a total of four doses. This induction phase is followed by maintenance therapy with nivolumab alone, given either as 240 mg intravenously every two weeks or 480 mg every four weeks, depending on clinical judgment and patient preference.
Here you can read about trials results.
What is Hepatocelular Carcinoma?
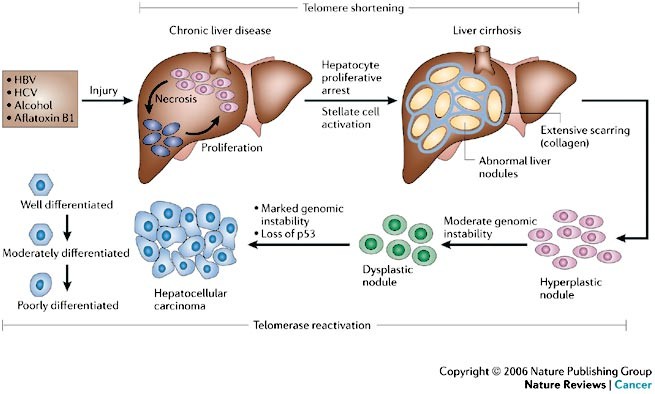
Liver cancer is a disease in which malignant (cancer) cells form in the tissues of the liver. It can either start in the liver itself (primary liver cancer) or spread to the liver from other parts of the body (secondary or metastatic liver cancer). The most common type of primary liver cancer is hepatocellular carcinoma (HCC), which begins in the main liver cells called hepatocytes.
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and ranks as the fifth most frequently diagnosed cancer worldwide. It is also the third leading cause of cancer-related deaths globally. The primary risk factors for HCC include chronic infections with hepatitis B virus (HBV) or hepatitis C virus (HCV), prolonged exposure to aflatoxins (toxins produced by certain fungi), alcoholic cirrhosis, and cirrhosis resulting from other causes.
When liver cancer was suspected, the evaluation typically began with a review of the patient’s medical history and a physical examination. The doctor asked about symptoms and checked for physical signs of liver disease, such as abdominal swelling or yellowing of the skin and eyes (jaundice).
If concerns remained after the exam, additional testing was ordered. Imaging tests—such as ultrasound, CT scans, or MRIs—were used to visualize the liver. These tests helped identify tumors, determine their size and location, and assess whether the cancer had spread. In some cases, the imaging results were detailed enough to make a diagnosis without the need for a biopsy, which was performed to confirm the presence of cancer. A small sample of liver tissue was collected using a needle, a laparoscope, or during surgery. The tissue was then examined under a microscope to look for cancer cells.
Blood tests also played a key role. They were used to detect tumor markers like alpha-fetoprotein (AFP), evaluate liver function, and check how well other organs were working. These results helped the medical team plan the most appropriate treatment and monitor the patient’s response over time.
Read more about liver cancer on OncoDaily.
Treatment for liver cancer depends on the type and stage of the disease. For hepatocellular carcinoma (HCC), early stages may be managed with surgery, such as partial hepatectomy or liver transplant. Other local treatments include ablation methods like radiofrequency ablation, cryoablation, or ethanol injection. Embolization techniques such as TACE or radioembolization may be used to block the tumor’s blood supply. Systemic options include targeted therapies (e.g., sorafenib, lenvatinib), immunotherapy (e.g., nivolumab, pembrolizumab), radiation, and chemotherapy.
How Nivolumab and Ipilimumab Work
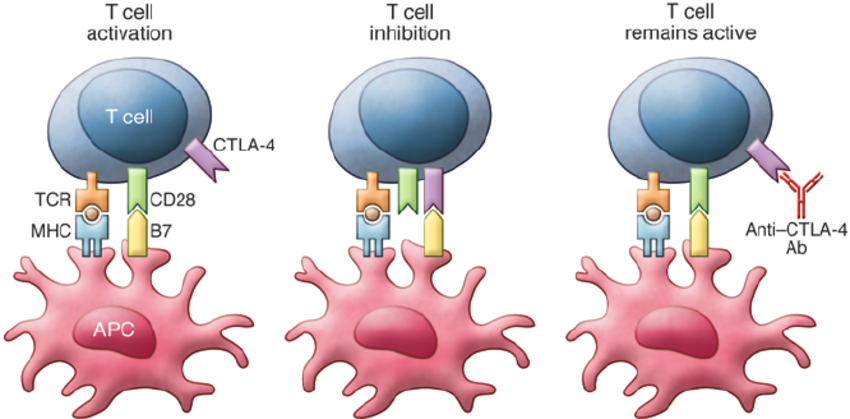
Ipilimumab is a monoclonal antibody that works by enhancing the body’s immune response against cancer cells. Its mechanism of action involves targeting CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), an immune checkpoint receptor expressed on T cells.
Under normal conditions, CTLA-4 functions as a negative regulator of T-cell activation. When T cells are activated by antigen-presenting cells (APCs), CTLA-4 is upregulated and competes with the costimulatory receptor CD28 for binding to B7 molecules (CD80/CD86) on APCs. Binding of CTLA-4 to B7 sends an inhibitory signal that dampens the T-cell response, maintaining immune homeostasis and preventing autoimmunity.
Ipilimumab blocks CTLA-4, thereby inhibiting this negative feedback mechanism. As a result, T-cell activation and proliferation are enhanced, especially cytotoxic T cells, which are capable of recognizing and killing tumor cells. This immune checkpoint blockade effectively amplifies antitumor immunity.
Nivolumab is a fully human IgG4 monoclonal antibody that functions as an immune checkpoint inhibitor targeting the programmed death-1 (PD-1) receptor on T cells.
PD-1 is an inhibitory receptor expressed on activated T cells, B cells, and natural killer (NK) cells. Its ligands, PD-L1 and PD-L2, are often expressed on tumor cells and tumor-infiltrating immune cells. When PD-1 binds to PD-L1 or PD-L2, it transmits an inhibitory signal that reduces T-cell proliferation, cytokine production, and cytotoxic activity. This pathway is exploited by tumors to evade immune surveillance by creating an immunosuppressive microenvironment.
Nivolumab binds to PD-1 and prevents its interaction with PD-L1 and PD-L2, thereby releasing the “brakes” on T cells. This blockade restores T-cell function, enhances their proliferation and cytokine release, and reactivates antitumor immune responses.
Read about Immunotherapy vs Chemotherapy on Oncodaily.
New treatment options for HCC
The phase III LEAP-012 trial evaluated the addition of lenvatinib and pembrolizumab to transarterial chemoembolization (TACE) in patients with unresectable, non-metastatic HCC. The combination significantly improved progression-free survival (PFS) to 14.6 months compared to 10.0 months with TACE alone (hazard ratio [HR] = 0.66; p=0.0002). While overall survival data are still maturing, the trend is promising.
Geneos Therapeutics is developing a personalized cancer vaccine that, when combined with pembrolizumab, has shown significant tumor shrinkage in approximately 30% of advanced HCC patients in early-phase trials.
Carisma Therapeutics, in collaboration with Moderna, has nominated its first in vivo CAR-Macrophage (CAR-M) candidate for HCC, marking progress in innovative immunotherapy approaches.
These advancements represent a significant shift in the management of unresectable HCC, offering new therapeutic options and hope for improved patient outcomes.
Written by Mariam Khachatryan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
