Tom Powles, Head of Solid Tumor Research at Barts Cancer Institute, spotlights key Genitourinary Oncology updates from ESMO25.
“Biomarkers in RCC have been disappointing. RNA immune and angio signatures were identified in Immotion150.
Cabozantinib + Nivolumab in RNA Cluster 1/2 (angio) in M1 RCC shows great response rates in these patients. We can do these difficult studies.”
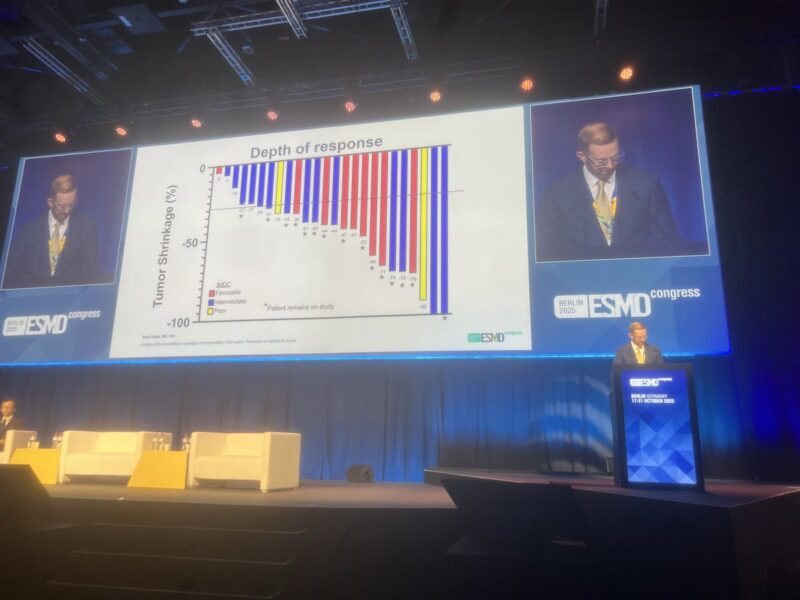
“Contemporary data for Levantinib + Everolimus is not robust. Levantinib/Everolimus vs Cabozantinib (n=90) equal PFS HR 0.51, OS HR 1.05, 67% G3/4 AEs. Wide confidence intervals, but the combo is active, but with high AEs and no OS, sequencing therapy remains important.”

“Renal cancer 1st line randomized PII KMUA3B at ESMO25 with Lenavatinib/Pembrolizumab as the control (hard to beat with RR>80%).
Lenavatinib/Pembrolizumab/Belzutifan showed good PFS (HR 0.45), which may lead to a positive RIII (LS12), which is ongoing. TIGIT and LAG3 were disappointing again.”
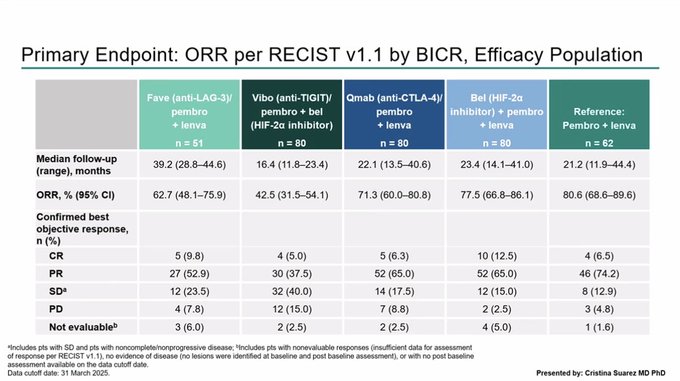
“Durvalumab/Tremelimumab vs Durvalumab (n=80) in pretreated clear cell renal cancer shows higher ORR for the combination but no clear OS benefit.
KIM-1 continues to be a useful prognostic marker. Enrichment in PD-L1 +ve was not apparent, but low numbers.”
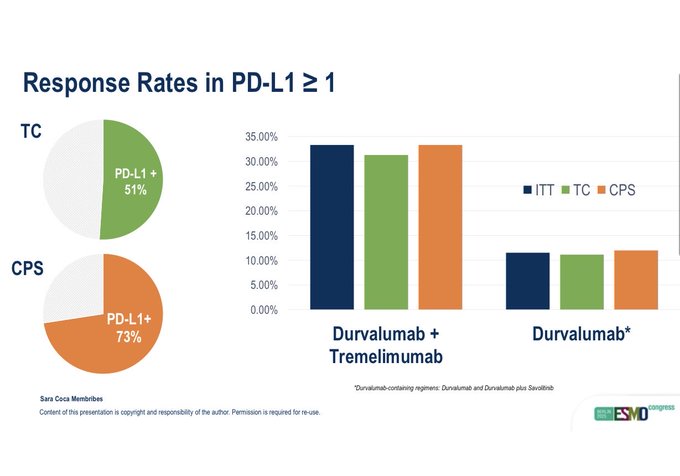
“NIAGARA QOL data shows perioperative Durvalumab with neoadjuvant chemotherapy has similar data to chem alone. This builds on the positive OS and EFS data, as well as no surgical tox. This approach is preferable to waiting for adjuvant IO. Early IO helps.”
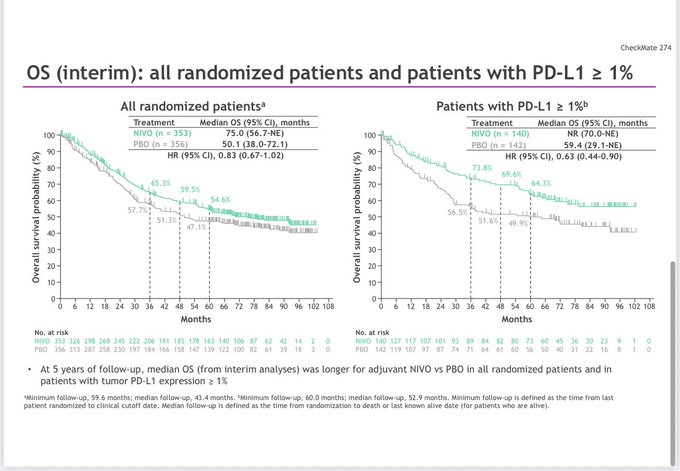
“Adjuvant Nivolumab remains positive for DFS HR-0.73 but still not +ve for OS 0.83 in operable UC. Enrichment occurred in the ctDNA +ves (DFS HR=0.3 and OS HR=0.44) as in IMvigor010. The ctDNA negatives were at much lower risk, didn’t seem to benefit from IO (HR-DFS 0.99, OS 0.87).”
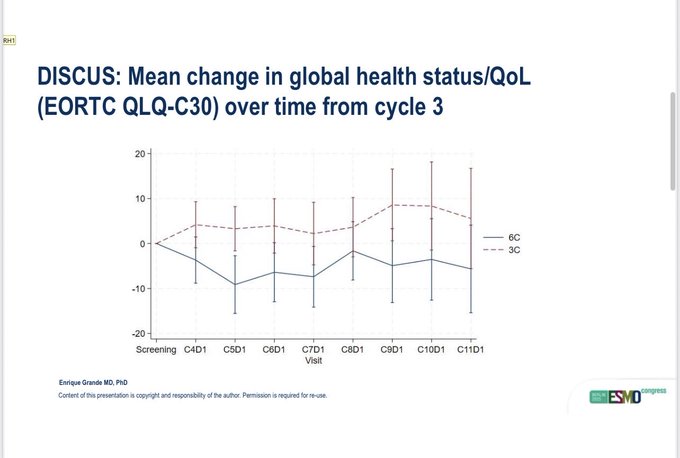
“DISCUS tested 3 vs 6 cycles of platinum chemotherapy followed by Avelumab in 1st line metastatic UC. QOL was the primary endpoint, showing better results with the 3-cycle arms. It also showed similar PFS, OS, and RR in the 2 arms with OS at 18 months from the start of chemo.”
“ALBAN, POTOMAC, and CREST (Atezolizumab/Sasalimab/in MIBC) show a lot of variability. It is not easy to conclude exactly which NMIBC patients benefit from ICI therapy. There is over-treatment, and it’s not without toxicity.”
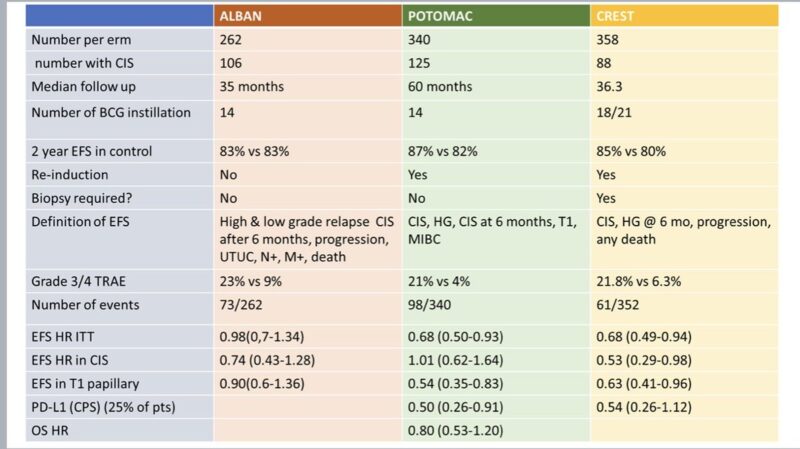
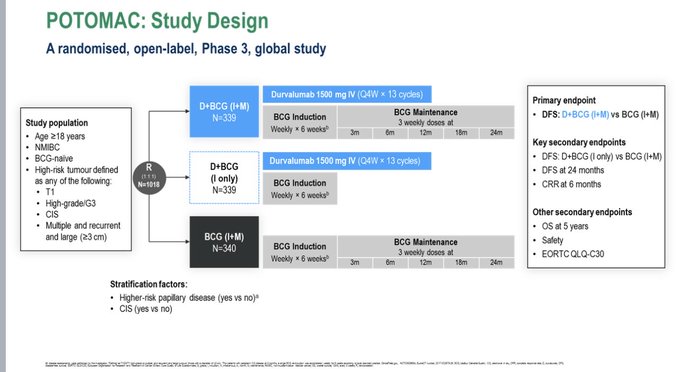
“POTOMAC (Durvalumab/BCG vs BCG in MIBC) shows delayed EFS (HR 0.69) – the same as Sasanlimab in the same setting.
There was no enrichment in the CIS population (unlike Sasalimab). Not all high-risk patients need PD(L)1 therapy, but it doesn’t look like we know who does.”
“ALBAN (Atezolizumab/BCG vs BCG in MIBC) did not improve EFS (HR 0.97), unlike Sasalimab and Durvalumab in a similar setting.
The drugs seem similar in metastatic UC, so was it trial design making it different from other ICIs?
EFS was defined differently.”

More posts featuring Tom Powles on OncoDaily.


