The 10th Anniversary Liquid Biopsy for Precision Oncology Summit will take place on February 3–5, 2026, in La Jolla, San Diego, California, marking a decade of collaborative progress in circulating biomarkers and their expanding role across oncology drug development. Organized by Hanson Wade, the meeting will be held at the San Diego Marriott La Jolla and is expected to bring together more than 250 leaders from biopharma, diagnostics, academia, clinical research, regulatory agencies, and payer communities.
Celebrating a Decade of Innovation in Liquid Biopsy
Over the last ten years, the field of liquid biopsy has reshaped how oncology teams understand tumor biology, therapeutic resistance, and real-time patient response. Liquid biopsies now enable identification of actionable mutations, dynamic treatment monitoring, and detection of minimal residual disease (MRD) with far greater sensitivity than traditional methods. These capabilities are enhancing clinical trial efficiency and improving therapeutic precision, while also raising complex questions about regulatory expectations, payer-relevant evidence, and demonstration of long-term clinical utility.
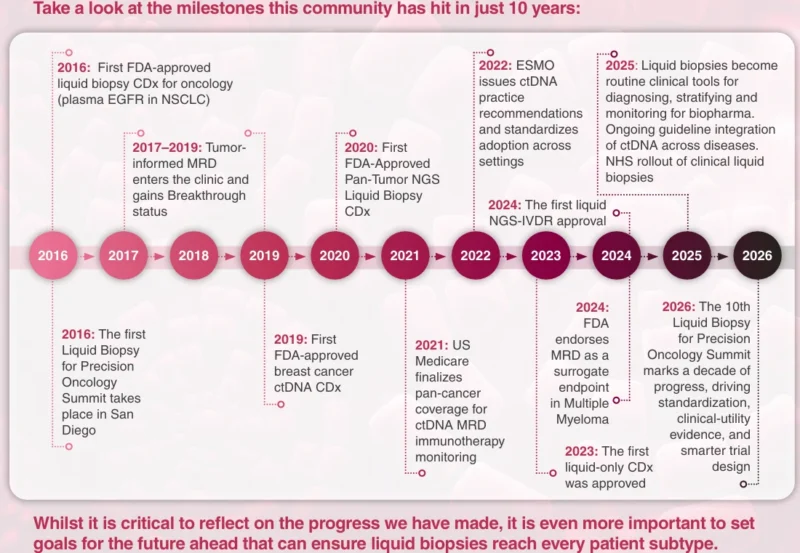
The anniversary summit reflects this evolution, advancing a shared goal: building consensus on how liquid-based biomarkers can be more effectively embedded into development workflows and clinical pathways.
A Cross-Sector Effort to Accelerate Precision Oncology
Regardless of disease indication or therapeutic modality, liquid biopsy is increasingly central to patient stratification, early relapse detection, and evaluation of surrogate endpoints. The 2026 summit will gather experts committed to addressing these needs by aligning translational, regulatory, diagnostic, and commercial strategies.
Sessions will explore how liquid biopsy can support adaptive trial designs, allow earlier efficacy assessment, and enable MRD-guided decision-making. Additional discussions will focus on regulatory harmonization to reduce delays, generation of real-world evidence that resonates with payers, and partnerships with diagnostic developers and CROs to de-risk implementation.
Shaping Evidence, Strategy, and Future Adoption
Attendees will hear updates on the use of ctDNA, circulating tumor cells (CTCs), and multi-omic biomarkers tailored to various therapeutic modalities. Emerging analytes and next-generation technologies—such as methylation profiling—will be highlighted for their ability to deliver deeper resolution into tumor heterogeneity, refine predictive biomarker strategies, and reduce late-stage trial failures.
The program also examines evolving pathways for validating MRD as a surrogate endpoint across hematologic malignancies and explores the landscape for achieving the first MRD-based indication in solid tumors. Presentations will cover how blood-based biomarkers are expanding into early detection, recurrence monitoring, and new disease areas, opening novel therapeutic and diagnostic opportunities.
Driving Collaboration for Scalable Precision Oncology
As liquid biopsy assumes a more influential role in drug development and clinical practice, consensus on evidence generation, regulatory readiness, reimbursement frameworks, and clinical integration remains essential. By bringing together leaders from all parts of the precision oncology ecosystem, the summit aims to equip participants with the insights and partnerships needed to enable more confident decision-making, faster development timelines, and more accessible precision therapies for patients with unmet needs.
Dates: February 3–5, 2026
Venue: San Diego Marriott La Jolla, La Jolla, CA
Organizer: Hanson Wade Limited
View the full agenda here.