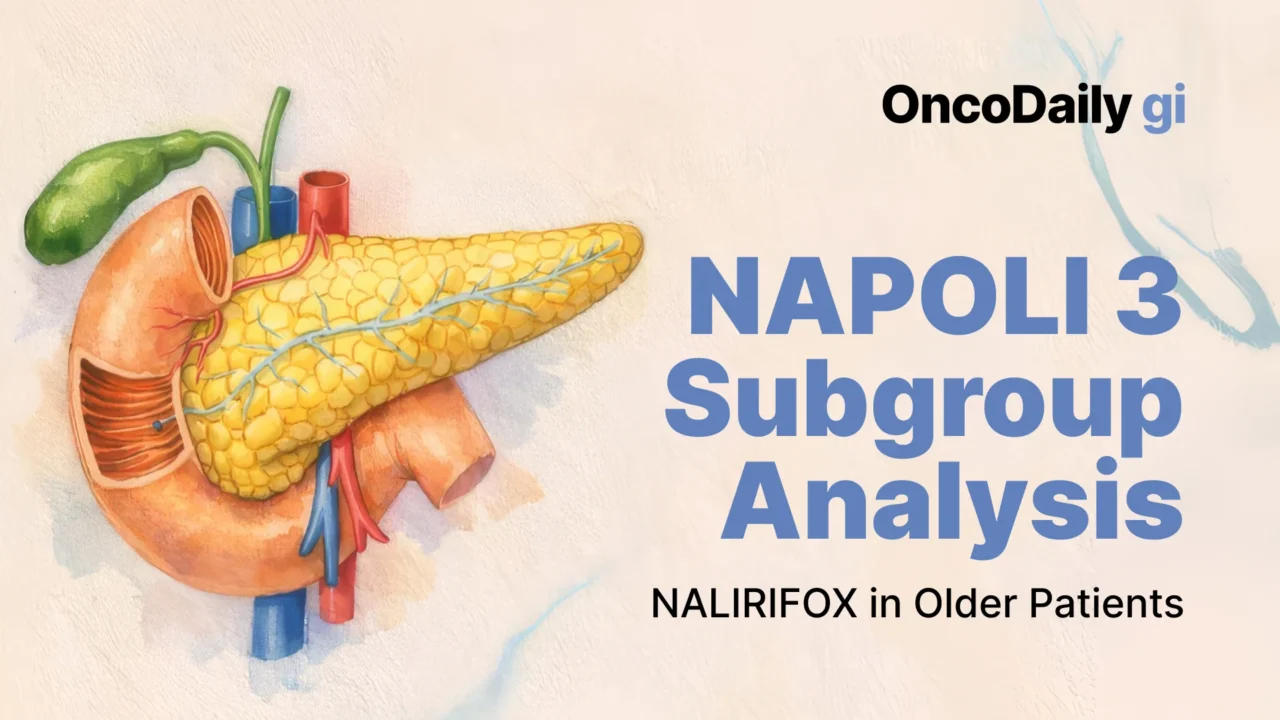
NAPOLI 3 expanded first-line options in metastatic pancreatic ductal adenocarcinoma by showing that NALIRIFOX (liposomal irinotecan + 5-FU/leucovorin + oxaliplatin) improves survival versus gemcitabine + nab-paclitaxel. This post hoc subgroup analysis focuses on older adults, comparing patients ≥70 vs <70 years, and finds that efficacy and tolerability were broadly consistent across ages—supporting intensive therapy for fit older patients rather than using age alone to limit treatment.
Title: NALIRIFOX versus nab-paclitaxel and gemcitabine in older patients with treatment-naive metastatic pancreatic cancer: a subgroup analysis of the pivotal NAPOLI 3 trial
Authors: T. Macarulla, R.Pazo Cid, M.S. Womack, A. Cubillo Gracian, A. Zervoudakis, H. Hatoum, V. Chung, A. Patel, A.S. Paulson, S. Pant, E.M. O’Reilly, R.A. Hubner, E. Van Cutsem, T. Bekaii-Saab, L. Zhang, J. Li, H. Chen, F. Maxwell, Z.A. Wainberg, D. Mel1isi
Published in Annals of Oncology, Feb. 2026
Background
The phase III NAPOLI 3 trial established NALIRIFOX (liposomal irinotecan + 5-fluorouracil/leucovorin + oxaliplatin) as a superior first-line option versus gemcitabine + nab-paclitaxel (Gem + NabP) for previously untreated metastatic pancreatic ductal adenocarcinoma (mPDAC). Because pancreatic cancer incidence peaks in older age groups and older adults are frequently underrepresented in intensive-regimen trials, this subgroup analysis asked a very practical question: does being ≥70 years old meaningfully change the efficacy or tolerability of NALIRIFOX in fit patients? The analysis is especially relevant because NAPOLI 3 had no upper age limit, enrolling patients up to 85 years, and therefore provides prospective trial data in an area often guided by assumptions rather than evidence.
Methods
This was a post hoc analysis of NAPOLI 3 comparing outcomes by age: ≥70 years versus <70 years. Endpoints included overall survival (OS), progression-free survival (PFS), objective response rate (ORR), duration of response (DoR), and safety (treatment-emergent adverse events, including grade 3–4 toxicities, serious TEAEs, treatment discontinuations, and deaths). Time-to-event outcomes were estimated using Kaplan–Meier methods, with hazard ratios (HRs) from Cox models. Importantly, the report notes that no statistical comparison was carried out for the age subgroup analysis and results should be interpreted descriptively.
Study Design
NAPOLI 3 was a randomized, open-label, phase III study conducted at 187 sites in 18 countries, enrolling adults (≥18 years) with previously untreated mPDAC and ECOG performance status 0–1. Patients were randomized 1:1 to:
- NALIRIFOX on days 1 and 15 of a 28-day cycle (liposomal irinotecan 50 mg/m², oxaliplatin 60 mg/m², leucovorin 400 mg/m², fluorouracil 2400 mg/m² as a 46-hour infusion), or
- Gem + NabP on days 1, 8, and 15 of a 28-day cycle (nab-paclitaxel 125 mg/m², gemcitabine 1000 mg/m²).
The intention-to-treat (ITT) population included 770 patients (NALIRIFOX n=383, Gem + NabP n=387). In this age analysis, 553 patients (71.8%) were <70 and 217 (28.2%) were ≥70. Among those receiving NALIRIFOX, 275 were <70 and 108 were ≥70.
Baseline characteristics were generally balanced across age groups within the NALIRIFOX arm, with two notable differences described: the ≥70 subgroup had a higher proportion with only one metastatic site, and a lower proportion with ECOG 0 (i.e., more ECOG 1) compared with <70—both clinically relevant because metastatic burden and baseline functional status can influence outcomes and treatment tolerance.
Results
In the safety population for NALIRIFOX, median treatment exposure was 5.8 months in patients <70 (range 0.3–23.1) versus 4.0 months in those ≥70 (range 0.1–23.2). Exposure in the overall NALIRIFOX safety cohort was 5.6 months (range 0.1–23.2). Shorter exposure in older patients can reflect multiple real-world factors (earlier discontinuation, progression, clinician caution), so it is meaningful that efficacy and key safety signals remained broadly consistent despite this.
Efficacy within the NALIRIFOX arm: older versus younger
Among patients treated with NALIRIFOX, survival outcomes were remarkably similar across age groups.
- For <70 years (n=275), median OS was 11.7 months (95% CI 10.3–12.8) and median PFS was 7.4 months (95% CI 6.0–8.7).
- For ≥70 years (n=108), median OS was 10.0 months (95% CI 7.9–11.8) and median PFS was 7.3 months (95% CI 5.6–9.2).
These figures align closely with the overall NALIRIFOX ITT population results previously reported (median OS 11.1 months, median PFS 7.4 months), supporting the paper’s main message that there was no clear evidence of an age-related decrement in NALIRIFOX benefit in patients fit enough for trial entry.
Tumor response patterns showed more separation by age than survival did. ORR with NALIRIFOX was 45.5% (95% CI 39.5–51.5) in <70, versus 32.4% (95% CI 23.7–42.1) in ≥70—a numeric reduction in response rate among older patients. However, the median DoR for NALIRIFOX was not inferior in older adults: 7.2 months in <70 (95% CI 5.7–7.6) versus 7.8 months in ≥70 (95% CI 3.7–12.9). This combination—lower ORR but similar durability among responders—helps explain why survival remained consistent across ages.
Comparative efficacy: NALIRIFOX versus Gem + NabP by age
The analysis also asked whether age changed the relative advantage of NALIRIFOX over Gem + NabP. It did not. Median OS favored NALIRIFOX in both subgroups:
- In <70, OS was 11.7 vs 9.6 months (HR 0.83, 95% CI 0.68–1.01).
- In ≥70, OS was 10.0 vs 8.7 months (HR 0.86, 95% CI 0.63–1.17).
PFS also favored NALIRIFOX across ages:
- In <70, PFS was 7.4 vs 5.6 months (HR 0.68, 95% CI 0.55–0.84).
- In ≥70, PFS was 7.3 vs 5.9 months (HR 0.72, 95% CI 0.51–1.02).
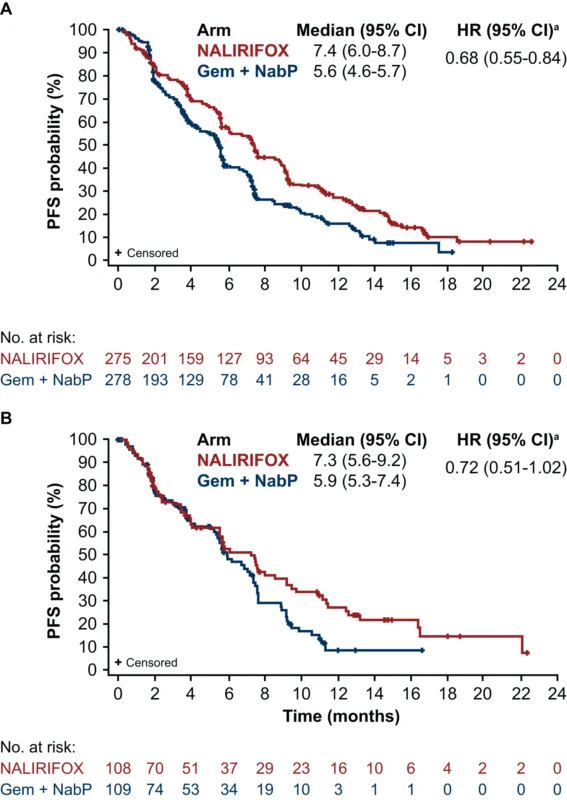
While some comparisons in smaller older subgroups did not reach statistical significance, the paper appropriately frames this as a sample-size and power issue rather than a clear loss of benefit with age.
Safety in older patients
Safety outcomes were broadly consistent by age, with no new safety signals for patients ≥70. Nearly all patients experienced TEAEs, as expected for intensive chemotherapy. For NALIRIFOX, grade ≥3 TEAEs were 86.9% in <70 and 87.4% in ≥70 (overall NALIRIFOX safety population 87.0%), showing no age-related inflation in severe toxicity rates.
Two hallmark toxicities for NALIRIFOX—diarrhea and neutropenia—were specifically detailed. Any-grade diarrhea occurred in 70.4% (<70) and 70.9% (≥70), while grade 3–4 diarrhea occurred in 19.1% (<70) and 23.3% (≥70), compared with 20.3% overall. For neutropenia (including neutrophil count decrease), grade 3–4 neutropenia was 24.0% in <70 and 21.4% in ≥70 (overall 23.2%). These numbers support the authors’ conclusion that toxicity burden did not meaningfully worsen in the older subgroup within this fit trial population.
Other clinically important safety endpoints were also reassuring. Serious TEAEs were somewhat higher numerically in older NALIRIFOX patients (59.2%) than younger (52.4%), but discontinuation rates did not rise with age: TEAEs leading to discontinuation were 33.7% (<70) versus 27.2% (≥70), with 31.9% overall. TEAEs leading to death were 6.7% (<70) and 3.9% (≥70), similar to the overall rates reported for the regimen.
Key findings
First, NALIRIFOX maintained clinically meaningful survival outcomes in older adults, with median PFS essentially identical between ≥70 and <70 (7.3 vs 7.4 months). Second, the relative benefit versus Gem + NabP was preserved in older patients, with OS and PFS hazard ratios in the same range as the overall trial. Third, toxicity did not escalate with age in this fit population: severe adverse event rates and discontinuations were similar, and no age-specific safety signal emerged. Finally, although ORR was numerically lower in ≥70, response durability remained comparable, supporting the survival consistency.
Conclusion
This subgroup analysis of NAPOLI 3 provides practical, trial-based reassurance that NALIRIFOX can be both effective and tolerable in patients aged ≥70 years with previously untreated mPDAC who are sufficiently fit for intensive therapy (ECOG 0–1). Median OS with NALIRIFOX was 10.0 months in ≥70 versus 11.7 months in <70, and median PFS was 7.3 versus 7.4 months, with comparative benefit versus Gem + NabP maintained across ages. Safety profiles were consistent, with grade 3–4 diarrhea around one-fifth of patients and grade 3–4 neutropenia around one-quarter, without an age-driven toxicity penalty.
The overarching clinical message is straightforward and evidence-based: age alone should not preclude intensive first-line therapy in older adults with mPDAC; treatment selection should be guided by functional status, comorbidities, and patient priorities—exactly the kind of decision this analysis helps clinicians make with more confidence.