LARA Trial was designed to address the persistent unmet need in recurrent gynaecological clear cell carcinoma, a rare and biologically distinct subtype of ovarian and endometrial cancer that is often resistant to standard chemotherapy. Conducted as a multicentre, single-arm, phase 2 study across tertiary centres in Singapore and South Korea, the trial explored a dual-pathway strategy combining PD-1 immune checkpoint inhibition with multi-kinase anti-angiogenic therapy, based on the unique molecular features and immunosuppressive tumour microenvironment characteristic of clear cell disease.
Background
Clear cell carcinomas arising from the gynaecological tract—including ovarian and endometrial clear cell carcinomas—represent an uncommon but clinically challenging subgroup, often characterized by relative resistance to standard chemotherapy in the recurrent setting and limited prospective evidence to guide systemic treatment.
In recurrent ovarian clear cell carcinoma, historical data suggest low objective response rates with chemotherapy (reported around 0–19%), underscoring a persistent unmet need. Biologically, clear cell tumors show features that support combined angiogenesis and immunotherapy approaches: upregulation of hypoxia-responsive and pro-angiogenic pathways (including VEGF signaling) and an immunosuppressive tumor microenvironment that may be partially reversible with anti-angiogenic therapy.
The LARA trial evaluated whether dual pathway targeting with pembrolizumab (PD-1 blockade) plus lenvatinib (multi-kinase anti-angiogenic inhibitor) could produce clinically meaningful activity in patients with recurrent gynaecological clear cell carcinoma (CCGC), including those previously treated with anti-angiogenic agents.
Methods and Study Design
LARA was a multicentre, single-arm, phase 2 trial conducted across three tertiary hospitals in Singapore and South Korea. The study used Simon’s two-stage minimax design to test whether the regimen could exceed an objective response rate (ORR) benchmark considered clinically meaningful for this population.
- The primary endpoint was investigator-assessed ORR within the first 24 weeks by RECIST v1.1 (with confirmation required on a subsequent scan at least one month later).
- Secondary endpoints included ORR by immune RECIST (iRECIST), clinical benefit rate (CBR; response or stable disease) at 24 weeks, progression-free survival (PFS), overall survival (OS), duration of response, and CA-125 biochemical response for evaluable ovarian cancer participants per GCIG criteria.
- Safety was assessed using CTCAE v5.0.
Eligible participants were adults (≥18 years) with histologically confirmed ovarian or endometrial CCGC that had progressed or recurred after at least one prior platinum-based chemotherapy line, ECOG performance status 0–1, and no prior immune checkpoint inhibitor exposure. Patients required measurable disease by RECIST v1.1 and adequate organ function, including controlled blood pressure. Importantly, all enrolled tumors were proficient mismatch repair and/or microsatellite stable, reflecting a population not enriched for MSI-H/dMMR biology.
Treatment
Patients received pembrolizumab 200 mg intravenously every 3 weeks plus lenvatinib 20 mg orally once daily, continued until progression, unacceptable toxicity, withdrawal of consent, or for a maximum of 2 years. Stepwise dose reductions were permitted for lenvatinib toxicity; pembrolizumab dose reduction was not planned, but treatment could be held or discontinued for suspected immune-related adverse events.
Analysis Sets and Follow-up
Among 30 screened patients, 27 received at least one dose of both agents (safety population). The modified intention-to-treat efficacy population included 25 patients who met protocol compliance criteria and had both baseline and post-baseline imaging at the first scheduled assessment. The data cutoff for activity and safety was March 19, 2025, with a median follow-up of 21.0 months (IQR 12.5–25.2).
Results
The median age was 52 years (IQR 40–66). Most participants had ovarian clear cell carcinoma (24/27; 89%), while a smaller subset had endometrial clear cell carcinoma (3/27; 11%). Patients were heavily pretreated for a rare cancer population: the median number of prior therapy lines was 2 (IQR 1–2), and 6/27 (22%) had received ≥3 prior lines. Prior anti-angiogenic therapy was common (17/27; 63%), predominantly bevacizumab (16/27; 59%). A clinically aggressive disease pattern was reflected by short platinum-free intervals: 16/27 (59%) had progression within <6 months after platinum, and 8/27 (30%) within ≤3 months.
Biomarker profiling highlighted typical “immune-cold” features for many cases. PD-L1 CPS was 0 in 22/26 (85%), CPS 1–9 in 2/26 (8%), and CPS ≥10 in 2/26 (8%). Among 19 patients with available tumor mutational burden (TMB) data, the median TMB was 3 mutations/Mb (IQR 1.3–5.3), and only 2/19 (11%) had TMB ≥10 mutations/Mb. Common alterations included ARID1A mutations (14/25; 56%) and PIK3CA mutations (12/25; 48%).
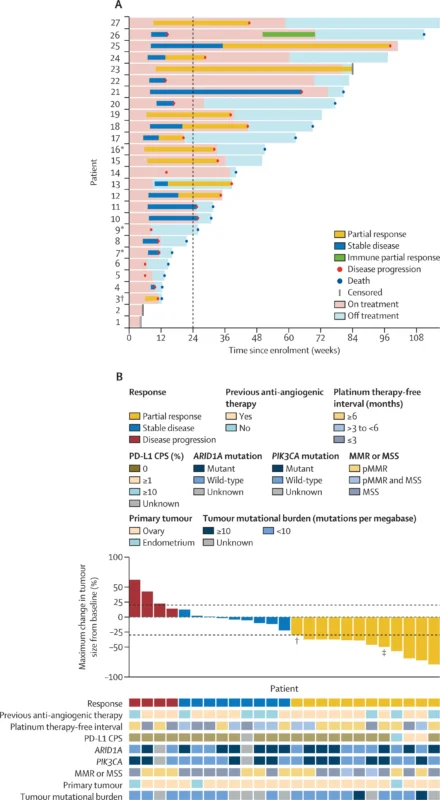
In the primary efficacy analysis (n=25), 10 patients achieved confirmed objective partial response within 24 weeks, yielding an ORR at 24 weeks of 40% (95% CI 21–61). Stable disease occurred in 10/25 (40%), producing a clinical benefit rate at 24 weeks of 84% (95% CI 64–96). ORR by iRECIST at 24 weeks matched RECIST at 40% (95% CI 21–61).
When responses occurring after 24 weeks were included (post-hoc), confirmed objective response was observed in 11/25 patients for an ORR of 44% (95% CI 24–65). By iRECIST, 12/25 achieved objective response (48% [95% CI 28–69]), driven in part by a delayed immune response pattern in one participant after initial iRECIST unconfirmed progression beyond week 24.
Notably, activity was maintained in patients previously exposed to anti-angiogenic therapy. Among 17 patients who had progressed on bevacizumab or another anti-angiogenic agent, the ORR was 47% (95% CI 23–72).
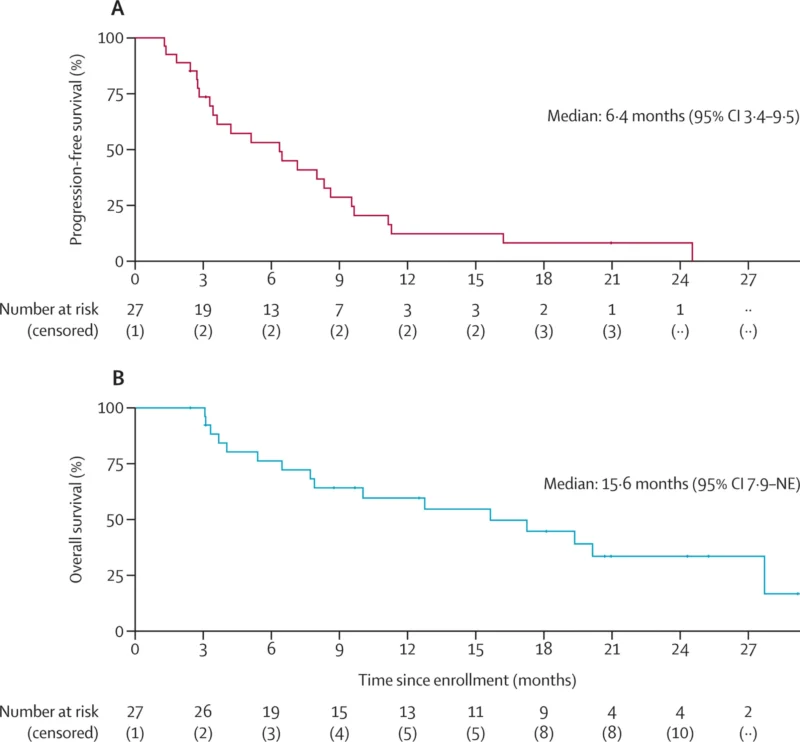
By data cutoff, 24/27 (89%) had progressed or died. Median PFS was 6.4 months (95% CI 3.4–9.5), and the PFS rate at 24 weeks was 53% (95% CI 37–77). Median duration of response was 6.6 months (95% CI 6.0–not available), and the median time to response was 2.8 months (post-hoc). Sixteen deaths (64%) were observed, all attributed to progressive disease, with median OS of 15.6 months (95% CI 7.9–not available). In exploratory subgroup reporting, patients with ovarian clear cell carcinoma had median PFS 6.5 months and OS 19.4 months (appendix-reported).
Responses occurred despite low PD-L1 expression and generally low TMB. Neither PD-L1 CPS nor TMB cleanly separated responders from non-responders in the reported analyses, and ARID1A/PIK3CA alterations (including co-mutations) were not associated with improved response or PFS in exploratory assessments.
Safety and Treatment Modifications
Treatment-related adverse events (TRAEs) occurred in 25/27 (93%), with grade 3–4 TRAEs in 14/27 (52%). The most common all-grade TRAEs included palmar-plantar erythrodysaesthesia (59%), hypertension (48%), anorexia (48%), oral mucositis (44%), fatigue (44%), and diarrhea (37%). Common grade 3–4 events included hypertension (22%) and less frequent hematologic and liver enzyme abnormalities (each around 7% for elevated AST/ALT and thrombocytopenia categories). Serious adverse events occurred in 5/27 (19%), including immune-related hepatitis in 2/27 (7%) and grade 3 immune-related primary adrenal insufficiency in 1/27 (4%).
No treatment-related deaths were reported.Dose modification was the rule rather than the exception: lenvatinib interruptions occurred in 25/27 (93%), and dose reductions or discontinuation in 24/27 (89%). The median lenvatinib dose by the end of treatment was 10 mg daily (range 4–20), and dose reduction did not appear to reduce ORR in post-hoc assessment.
Key findings
- ORR 40% at 24 weeks (RECIST) in evaluable patients (10/25), with CBR 84% (response or stable disease).
- Durable signal of activity even in a high-risk cohort: 63% had prior anti-angiogenic therapy and 59% had platinum-free interval <6 months.
- Median PFS 6.4 months and median OS 15.6 months at ~21 months median follow-up.
- Toxicity was manageable but dose-intense, with 52% grade 3–4 TRAEs and frequent lenvatinib dose interruptions/reductions (93%/89%).
- Responses occurred largely independent of classic immunotherapy biomarkers: most tumors had PD-L1 CPS 0 (85%) and low median TMB (3 mut/Mb).
Conclusion
The phase 2 LARA trial demonstrates that pembrolizumab plus lenvatinib can deliver promising anti-tumor activity in recurrent gynaecological clear cell carcinoma, a setting where chemotherapy responses are historically limited and prospective evidence is scarce. In a predominantly ovarian clear cell cohort with frequent prior bevacizumab exposure and a high rate of platinum-resistant behavior, the combination achieved a 24-week objective response rate of 40% and a clinical benefit rate of 84%, with median PFS of 6.4 months and median OS of 15.6 months at the time of analysis. Toxicities were consistent with known profiles of PD-1 plus multi-kinase anti-angiogenic therapy and were largely manageable through supportive care and substantial lenvatinib dose modification.
Importantly, responses were observed even when PD-L1 expression was absent and tumor mutational burden was low, suggesting that the combination may extend potential immunotherapy benefit beyond traditional biomarker-selected populations. Overall, these findings support further evaluation of pembrolizumab–lenvatinib in randomized controlled trials to clarify comparative benefit, refine patient selection, and define its role alongside other emerging immunotherapy combinations in clear cell gynecologic cancers.


