KRAS G12D pancreatic cancer is recognized as the earliest and most prevalent oncogenic driver in pancreatic ductal adenocarcinoma (PDAC), present in more than 90% of cases and detectable in precursor lesions such as pancreatic intraepithelial neoplasia (PanIN). Although KRAS-driven activation of MAPK and PI3K pathways is well established in promoting epithelial transformation, its role in shaping the tumor microenvironment at the pre-invasive stage has remained unclear. A recent translational study published in Molecular Cancer investigated whether KRAS<sup>G12D</sup> activation in pancreatic ductal cells initiates inflammatory niche programming before invasive carcinoma develops, focusing on TNFα-mediated signaling, immune exclusion, and pancreatic stellate cell activation across organoid models, murine systems, and human precursor lesions.
Background
Pancreatic ductal adenocarcinoma (PDAC) is characterized by early activation of oncogenic KRAS, most commonly the KRAS<sup>G12D</sup> mutation, present in more than 90% of tumors. While KRAS-driven signaling through MAPK and PI3K pathways is known to initiate epithelial transformation, less is understood about how mutant KRAS shapes the tumor microenvironment before invasive cancer develops. PDAC is also defined by dense desmoplasia, inflammatory fibroblast activation, and profound T-cell exclusion—features traditionally thought to arise later in tumor evolution. The current study, published in Molecular Cancer, investigates whether oncogenic KRAS in pancreatic ductal cells can actively reprogram the surrounding niche during the earliest stages of neoplastic transformation.
Methods
The investigators used a human embryonic stem cell–derived pancreatic duct-like organoid (PDLO) model engineered with inducible KRAS<sup>G12D</sup>. This system allowed time-resolved evaluation of epithelial and microenvironmental changes following controlled activation of oncogenic KRAS.
Key experimental approaches included:
- Single-cell RNA sequencing (scRNA-seq) and ATAC-seq performed at multiple time points (day 1, day 3, and day 7 post-induction) to capture transcriptional and chromatin accessibility changes.
- Secretome analysis to identify epithelial-derived cytokines and ligands.
- Cell–cell communication modeling using CellChat and machine learning classifiers.
- T-cell migration assays to assess immune exclusion.
- Pancreatic stellate cell (PSC) co-culture experiments to evaluate fibroblast activation.
- In vivo validation using KRAS-driven KC mice (Ptf1a-Cre; Kras<sup>G12D</sup>).
Human validation through RNAscope analysis of pancreatic intraepithelial neoplasia (PanIN) samples and cyst fluid TNFα measurement in a prospective cohort of 80 patients with intraductal papillary mucinous neoplasms (IPMN).
Study Design
This was a translational, mechanistic study integrating in vitro organoid modeling, in vivo mouse validation, and human clinical samples. The design focused on early time points following KRAS activation to define pre-invasive changes.
The central hypothesis was that epithelial KRAS activation initiates a secretory inflammatory program that alters immune cell behavior and fibroblast phenotype prior to overt carcinoma formation.
Primary endpoints included:
- Identification of KRAS-dependent ligands driving epithelial–niche signaling.
- Quantification of immune cell migration changes.
- Characterization of fibroblast polarization states.
- Correlation of TNFα expression with dysplasia grade in human samples.
Results
Within 24 hours of KRASG12D induction, scRNA-seq demonstrated activation of MAPK and PI3K signaling pathways. By day 3 and day 7, there was significant upregulation of inflammatory pathways, particularly TNFα/NF-κB signaling.
Chromatin accessibility analyses confirmed enrichment of NF-κB binding motifs, indicating coordinated transcriptional activation. Importantly, these changes preceded any invasive morphological phenotype.Secretome profiling and CellChat modeling ranked TNFα among the highest-scoring ligands mediating epithelial–microenvironment communication. TNFα expression was KRAS-dependent and increased progressively over time.
Functional assays demonstrated:
- Conditioned media from KRAS-activated organoids significantly reduced CD8+ T-cell migration.
- Recombinant TNFα reproduced the T-cell exclusion phenotype.
- Neutralization of TNFα with infliximab restored T-cell migration.
TNFα exposure converted pancreatic stellate cells into inflammatory cancer-associated fibroblast (iCAF)-like states, characterized by elevated IL-6 and chemokine expression.
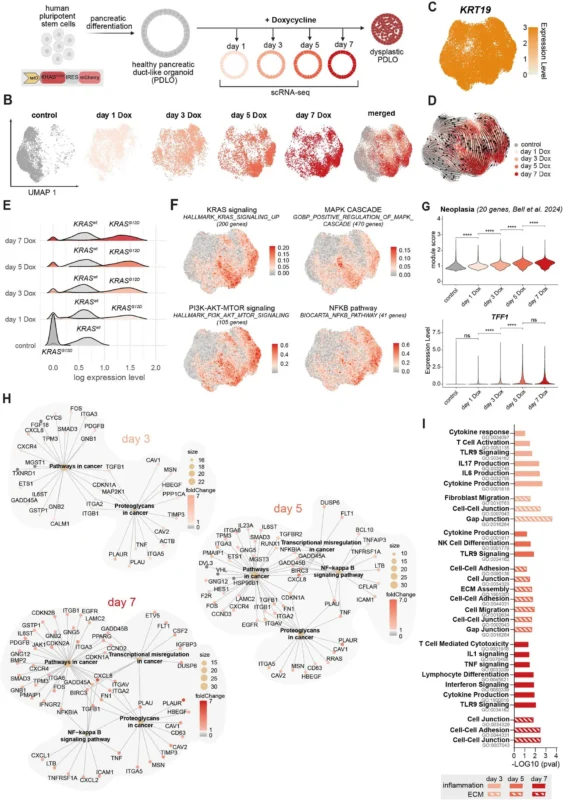
These findings establish a KRAS → NF-κB → TNFα signaling axis driving early niche remodeling.
Co-culture experiments showed that KRAS-driven epithelial cells promoted PSC activation, increasing extracellular matrix–related gene expression and inflammatory cytokines. This mirrors the desmoplastic reaction observed in PDAC.
In Ptf1a-Cre; Kras G12D mice, epithelial TNFα expression increased progressively from low-grade PanIN to high-grade PanIN lesions. This temporal association supports a role for TNFα in early tumor evolution rather than late-stage progression only.
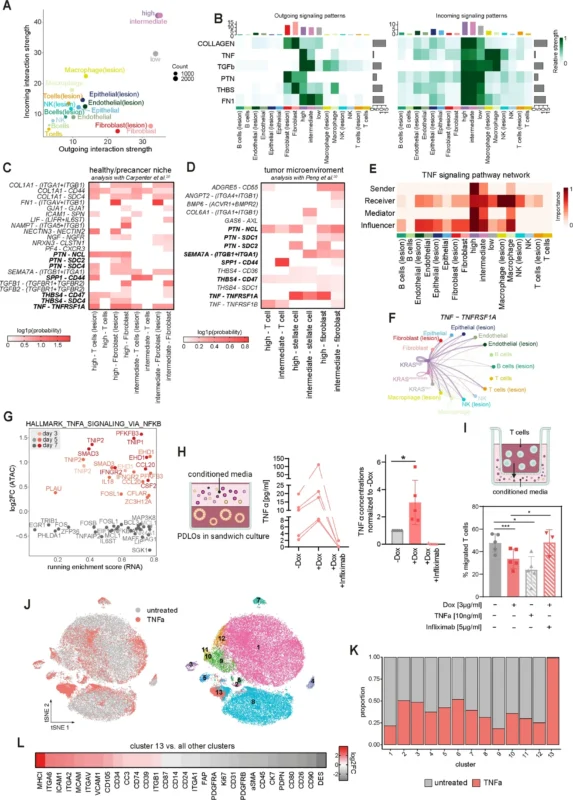
RNAscope analysis of human PanIN samples confirmed epithelial TNFα expression in dysplastic ducts. In the prospective IPMN cohort (n=80), cyst fluid TNFα concentrations increased stepwise with dysplasia severity, from low-grade dysplasia to high-grade dysplasia and invasive carcinoma. This quantitative gradient supports TNFα as a potential biomarker for neoplastic progression.
Key Findings
- Oncogenic KRAS<sup>G12D</sup> induces inflammatory transcriptional programs within 1–7 days of activation.
- TNFα is a central epithelial-derived ligand driving microenvironmental remodeling.
- KRAS-induced TNFα reduces T-cell migration and promotes immune exclusion.
- TNFα converts pancreatic stellate cells into inflammatory CAF phenotypes.
- TNFα expression increases with dysplasia grade in mouse and human samples.
- Cyst fluid TNFα levels correlate with IPMN severity in a cohort of 80 patients.
Conclusion
This study provides mechanistic evidence that oncogenic KRAS<sup>G12D</sup> initiates early inflammatory niche programming in pancreatic tumorigenesis. Through NF-κB–dependent TNFα secretion, mutant epithelial cells actively reshape their microenvironment by suppressing T-cell migration and activating inflammatory fibroblast phenotypes. Importantly, these alterations occur before invasive carcinoma develops, reframing pancreatic cancer as a coordinated epithelial–microenvironmental process from its earliest stages.
The translational validation in KC mice and human IPMN samples strengthens the biological relevance of these findings. Elevated cyst fluid TNFα levels across dysplasia grades suggest potential clinical utility in early detection and risk assessment.
By defining a KRAS–TNFα axis as an early driver of immune exclusion and stromal activation, this work shifts the paradigm of PDAC evolution and opens avenues for preventive and interception strategies targeting the tumor niche before invasive disease becomes established.


