JUPITER-06 is a randomized, double-blind, phase III trial evaluating toripalimab combined with paclitaxel and cisplatin as first-line treatment for advanced esophageal squamous cell carcinoma. The final analysis confirms a durable survival benefit with immunochemotherapy and highlights the value of refined genomic biomarkers, beyond PD-L1 and conventional TMB, in identifying patients most likely to benefit from treatment.
Background
Esophageal squamous cell carcinoma (ESCC) remains one of the most lethal gastrointestinal malignancies worldwide, with the majority of patients diagnosed at an advanced or metastatic stage. Although first-line immunochemotherapy with PD-1 inhibitors plus platinum-based chemotherapy has become the standard of care, survival outcomes remain highly heterogeneous. While a subset of patients achieves durable benefit, many experience early progression and limited survival despite treatment. Conventional biomarkers such as PD-L1 expression and tumor mutational burden (TMB) have shown inconsistent predictive value in the immunochemotherapy setting, underscoring the need for more refined biomarkers.
The phase III JUPITER-06 trial previously demonstrated that adding toripalimab, a humanized anti-PD-1 antibody, to paclitaxel plus cisplatin significantly improved progression-free and overall survival in untreated advanced ESCC. The present analysis reports the final overall survival results and provides an extensive exploratory genomic biomarker evaluation, aiming to identify molecular features associated with long-term benefit from immunochemotherapy.
Methods
JUPITER-06 enrolled patients with histologically confirmed, unresectable locally advanced, recurrent, or metastatic ESCC who had not received prior systemic therapy for advanced disease. Patients were randomized in a 1:1 ratio to receive toripalimab or placebo in combination with paclitaxel and cisplatin every three weeks for up to six cycles, followed by maintenance toripalimab or placebo.
Overall survival (OS) and progression-free survival (PFS) were co-primary endpoints, assessed by blinded independent central review.
Whole-exome sequencing was successfully performed on pretreatment tumor samples from 486 patients, enabling comprehensive genomic analyses including copy number alteration–corrected TMB (ccTMB), the esophageal cancer genome-based immuno-oncology classification (EGIC), and exploratory pathway-level alterations.
Study Design
This was a multicenter, randomized, double-blind, placebo-controlled phase III trial conducted across 72 centers in China. A total of 514 patients were randomized, with 257 patients assigned to each treatment arm. Eligible patients were aged 18–75 years, had an ECOG performance status of 0–1, and measurable disease per RECIST v1.1. The final OS analysis was performed with a data cutoff of February 23, 2023, after a median follow-up of 14.2 months. Genomic biomarker analyses were conducted in a predefined biomarker-evaluable population using high-quality whole-exome sequencing data.
Results
At the final analysis, 72.4% of patients in the intention-to-treat population had died. Toripalimab plus chemotherapy resulted in a statistically significant and clinically meaningful improvement in OS compared with chemotherapy alone. Median OS was 17.7 months (95% CI 14.6–20.8) in the toripalimab arm versus 12.9 months (95% CI 11.6–14.1) in the placebo arm, corresponding to a 28% reduction in the risk of death (HR 0.72; 95% CI 0.58–0.88; P = 0.002).
Landmark analyses showed consistent long-term benefit:
- 1-year OS: 64.4% vs 54.5%
- 2-year OS: 39.1% vs 27.1%
- 3-year OS: 29.7% vs 19.9%
favoring toripalimab plus chemotherapy.
With extended follow-up, the PFS benefit remained significant. Median PFS was 7.0 months with toripalimab plus chemotherapy versus 5.6 months with chemotherapy alone (HR 0.61; 95% CI 0.49–0.74; P < 0.0001), confirming the durability of disease control.
Conventional Biomarkers
Neither PD-L1 expression (by CPS or TPS) nor total or clonal TMB reliably predicted OS benefit from immunochemotherapy. Survival benefit with toripalimab was observed across PD-L1 subgroups, with no significant interaction detected, highlighting the limited discriminatory value of PD-L1 in this setting.
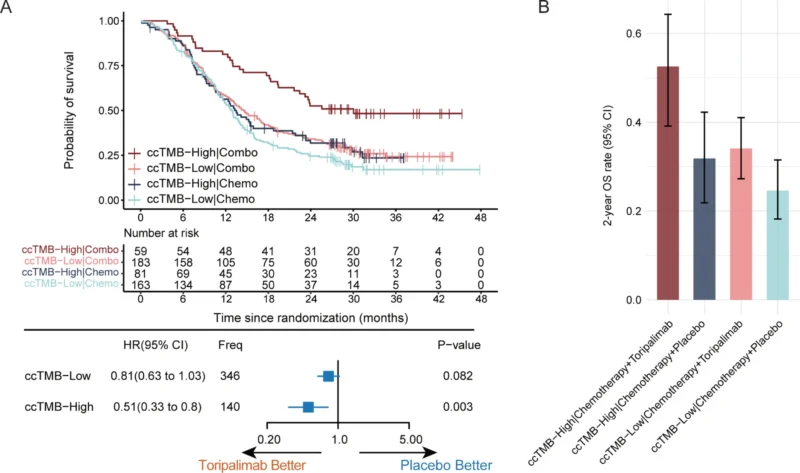
ccTMB and Long-Term Survival
Copy number alteration–corrected TMB (ccTMB) emerged as a robust predictive biomarker. Using the predefined 70th percentile cutoff, 28.8% of patients were classified as ccTMB-high. In this subgroup, toripalimab plus chemotherapy achieved a median OS of 30.0 months, compared with 13.5 months for chemotherapy alone (HR 0.51; P = 0.003). The 3-year OS rate reached 48.3% in ccTMB-high patients receiving toripalimab, substantially higher than any other subgroup. In contrast, patients with low ccTMB derived only marginal benefit.
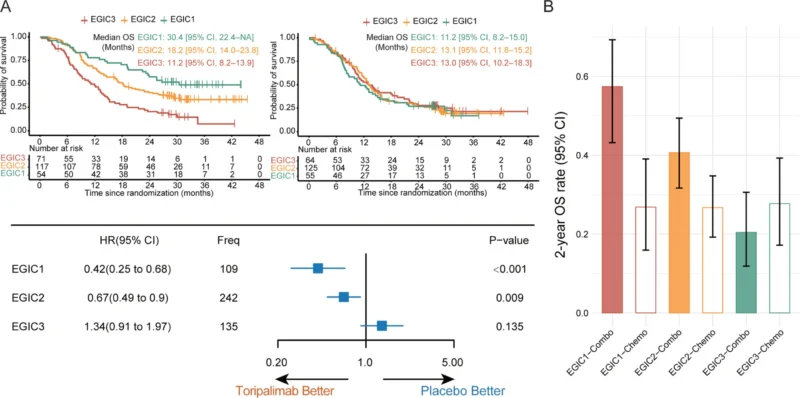
EGIC Classification
The EGIC scheme, integrating immunogenic and oncogenic features, further stratified patients into three prognostic groups. EGIC1 patients experienced the greatest benefit from immunochemotherapy (HR 0.42), EGIC2 patients showed intermediate benefit (HR 0.61), while EGIC3 patients derived no significant OS benefit. Under toripalimab-based therapy, median OS was 30.4 months for EGIC1, 18.2 months for EGIC2, and 11.2 months for EGIC3, demonstrating clear prognostic separation.
Oncogenic Pathway Alterations
Exploratory analyses revealed that loss-of-function alterations in the SWI/SNF chromatin remodeling complex (including ARID1A, SMARCA4, ARID2, and PBRM1) were associated with significantly improved long-term survival under immunochemotherapy, with a 2-year OS rate of 66.7%. Conversely, activation of cell cycle pathways (present in 72.6% of patients) and WNT signaling alterations (18.5%) were linked to reduced benefit from toripalimab. These findings suggest biologically distinct mechanisms of resistance and sensitivity.
Key Findings
- Toripalimab combined with paclitaxel and cisplatin provides a durable and clinically meaningful overall survival benefit as first-line treatment for patients with advanced esophageal squamous cell carcinoma.
- Conventional biomarkers, including PD-L1 expression and standard tumor mutational burden, were not sufficient to identify patients with long-term survival benefit from immunochemotherapy.
- Copy number alteration–corrected TMB (ccTMB) and the esophageal cancer genome-based immuno-oncology classification (EGIC) reliably stratified patients according to magnitude and durability of survival benefit.
- Loss-of-function alterations in the SWI/SNF chromatin remodeling complex were associated with enhanced efficacy of immunochemotherapy and improved long-term survival.
- Activation of cell cycle and WNT signaling pathways correlated with reduced benefit and potential resistance to toripalimab-based immunochemotherapy.
Conclusion
The final overall survival results of JUPITER-06 establish toripalimab plus chemotherapy as an effective and durable first-line treatment for advanced ESCC. Beyond confirming survival benefit, this study delivers important translational insights by demonstrating that refined genomic biomarkers can reliably distinguish patients with long-term benefit from those with limited response.
ccTMB and EGIC represent clinically relevant tools for precision immunotherapy, while pathway-specific alterations highlight opportunities for rational combination approaches. Together, these findings move the field closer to biomarker-driven treatment selection and improved outcomes in esophageal squamous cell carcinoma.


