GCD vs GCS has become a central question in the evolving treatment landscape of advanced biliary tract cancer, where survival remains poor and therapeutic decisions increasingly hinge on balancing efficacy, durability of response, toxicity, and patient-specific factors. With chemoimmunotherapy now widely adopted following the TOPAZ-1 trial and triplet chemotherapy gaining traction in Asia after the MITSUBA study, clinicians face two validated—but mechanistically distinct—first-line options.
This real-world investigation provides a direct comparison of durvalumab-based chemoimmunotherapy (GCD) versus gemcitabine/cisplatin/S-1 (GCS), evaluating survival outcomes, duration of response, safety, and genomic patterns that may eventually guide more personalized treatment selection.
Title: Durvalumab plus gemcitabine-cisplatin versus S-1 plus gemcitabine-cisplatin in advanced biliary tract cancer: a comparative study
Authors: T. Satake, M. Sasaki, H. Imaoka , S. Taro, K. Inoue, T. Taira, G. Igarashi, M. Amisaki, H. Takahashi, S. Mitsunaga, M. Ikeda
Background
Biliary tract cancer (BTC) is a rare, heterogeneous malignancy that includes intrahepatic and extrahepatic cholangiocarcinoma, gall-bladder cancer, and ampullary cancer. It is often diagnosed at an advanced stage and carries a poor prognosis, with 5-year survival rates of about 5% for intrahepatic cholangiocarcinoma and around 2% for extrahepatic cholangiocarcinoma and gall-bladder cancer. Even in resectable disease, more than half of patients relapse.
The combination of gemcitabine and cisplatin (GC) was long considered the standard first-line regimen, until phase III trials such as TOPAZ-1 and KEYNOTE-966 showed that adding an immune checkpoint inhibitor—durvalumab or pembrolizumab—modestly but significantly improved overall survival (OS). In parallel, in Japan, triplet chemotherapy with gemcitabine, cisplatin, and S-1 (GCS) demonstrated superiority over GC in the MITSUBA trial and is recognized in Pan-Asian ESMO guidelines as another standard first-line option.
However, no trials have directly compared chemoimmunotherapy with triplet chemotherapy in BTC, and biomarkers to guide treatment selection are lacking. This single-center retrospective study addressed that gap by comparing real-world efficacy, safety, and genomic correlates of GCD versus GCS.
Methods and Study Design
This was a single-center, retrospective cohort study conducted at the National Cancer Center Hospital East (Japan). Consecutive patients with advanced or recurrent BTC treated between November 2018 and August 2024 were included if:
- They had intrahepatic, perihilar, distal cholangiocarcinoma, gall-bladder carcinoma, or ampullary cancer.
- They had not received prior palliative systemic therapy for BTC (perioperative systemic therapy was allowed).
- They received at least one cycle of either GCD or GCS as first-line therapy.
The primary endpoint was overall survival (OS) from treatment initiation. Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), duration of response (DoR), and safety.
Tumor response was assessed using RECIST v1.1. Adverse events (AEs) were graded with CTCAE v5.0. Multivariable Cox regression was used to explore baseline predictors of OS. Comprehensive genomic profiling (CGP) was performed in a subset using tissue- or blood-based next-generation sequencing panels; only pathogenic/likely pathogenic alterations were analyzed.
Treatment Regimens
- GCD (durvalumab + gemcitabine/cisplatin)- durvalumab 1500 mg IV plus gemcitabine 1000 mg/m² and cisplatin 25 mg/m² on days 1 and 8 of a 21-day cycle, for up to eight cycles.
- Maintenance durvalumab 1500 mg IV every 4 weeks continued until progression or unacceptable toxicity.
- GCS (S-1 + gemcitabine/cisplatin)- gemcitabine 1000 mg/m² and cisplatin 25 mg/m² IV on day 1 of a 14-day cycle, plus oral S-1 twice daily for 7 consecutive days.Cisplatin was limited to 16 cycles; gemcitabine and S-1 were continued beyond that until progression or toxicity.S-1 dose was BSA-adjusted (80–120 mg/day).
A total of 265 patients were included, with 97 receiving GCD (from December 2022 onward) and 168 receiving GCS (from November 2018 onward). Key baseline features were well balanced between groups. The median age was 71 years overall (72 in the GCD group vs 71 in the GCS group), and 60.4% of patients were male (59.8% vs 60.7% in GCD and GCS, respectively). An ECOG performance status of 0 was observed in 72.2% of patients in the GCD group and 62.5% in the GCS group.
The most common primary tumor site was the gall-bladder, followed by intrahepatic and perihilar bile duct tumors. Metastatic disease was present in more than half of patients in both groups (54.6% in GCD vs 58.3% in GCS), and CA19-9 levels as well as the need for biliary drainage were similar between the two cohorts.
Results
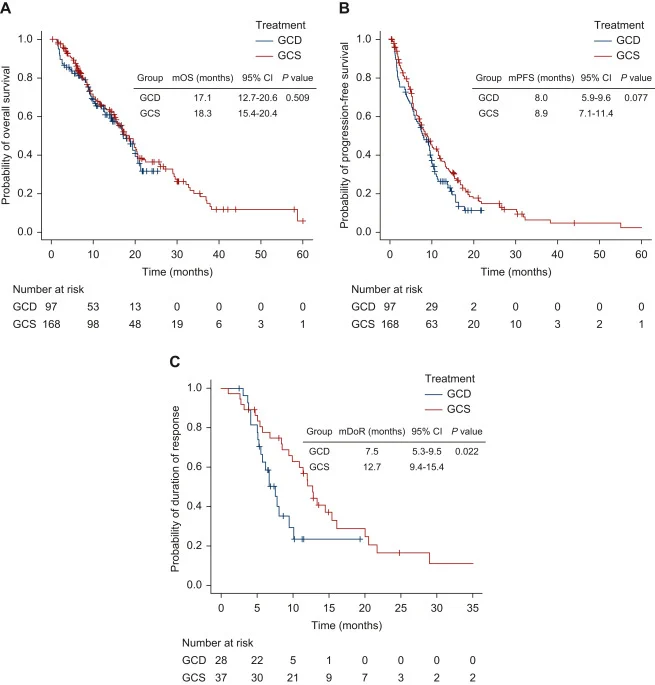
At data cut-off (31 March 2025), median follow-up was 10.7 months in the GCD group and 13.0 months in the GCS group.
- median OS for GCD was 17.1 months (95% CI 12.7–20.6)
- median OS for GCS was 18.3 months (95% CI 15.4–20.4)
- No statistically significant difference (P = 0.509).
- 12-month OS: 65.4% (GCD) vs 65.7% (GCS).
- median PFS for GCD 8.0 months (95% CI 5.9–9.6)
- median PFS for GCS 8.9 months (95% CI 7.1–11.4)
- trend favoring GCS but not statistically significant (P = 0.077).
In multivariable analysis, ECOG-PS ≥1 (HR 2.21; P < 0.001) and metastatic disease versus locally advanced (HR 1.71; P = 0.036) were independent adverse predictors of OS. Treatment type (GCD vs GCS) was not an independent driver of survival (HR for GCS vs GCD 0.75; 95% CI 0.51–1.09; P = 0.132).
Response and Duration of Response. Investigator-assessed tumor responses were similar:
- ORR: 28.9% (GCD) vs 22.0% (GCS), P = 0.237.
- DCR: 69.1% vs 74.4%, P = 0.392.
Complete responses were rare in both arms (1 patient each).
However, duration of response differed markedly:
- Median DoR for GCD was 7.5 months (95% CI 5.3–9.5)
- Median DoR for GCS was 12.7 months (95% CI 9.4–15.4)
- Statistically significant advantage for GCS (P = 0.022).
Proportion of responders with ongoing response:
- At ≥6 months: 62.6% (GCD) vs 77.7% (GCS)
- At ≥9 months: 35.2% vs 68.8%
- At ≥12 months: 23.5% vs 53.7%
- Conversion surgery was rare but similar (3.1% GCD vs 2.4% GCS).
Safety
Almost all patients experienced some AE:
- Any-grade AE: 96.9% (GCD) and 96.4% (GCS).
- Grade ≥3 AEs were more frequent with GCD: 66.0% vs 48.8% in GCS.
Immune-mediated AEs occurred only in the GCD group by design:
- Any-grade imAEs: 23.7%
- Grade ≥3 imAEs: 5.2%
- Most common imAEs: skin rash (9.3%), hyperthyroidism (4.1%), hypothyroidism (3.1%).
Treatment discontinuation due to AEs was similar (8 patients in each group). No treatment-related deaths occurred. Dose reductions were required in about two-thirds of patients in both groups; relative dose intensities for gemcitabine and cisplatin were comparable.
Genomic Profile
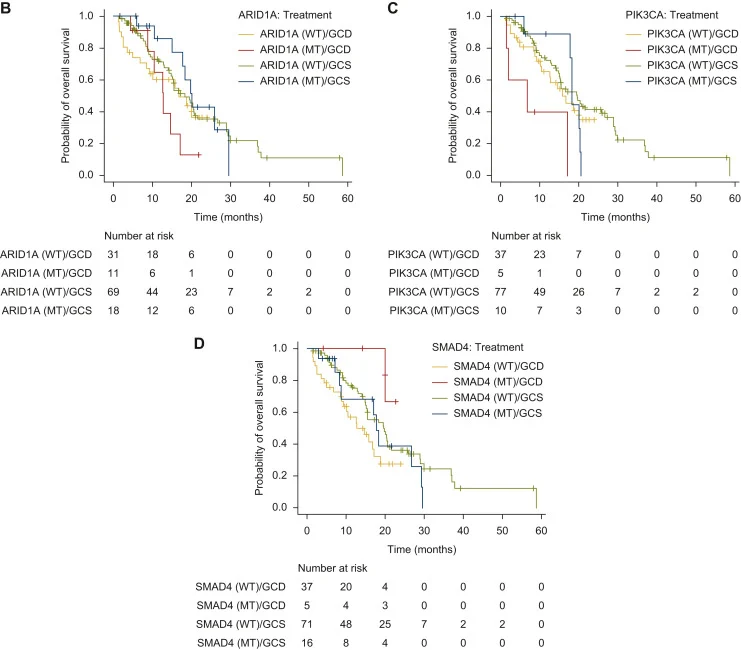
CGP was available for 129 patients (48.7%). Frequent alterations included TP53 (54.3%), KRAS (24.0%), ARID1A (22.5%), CDKN2A/B (16.3%), SMAD4 (16.3%), ERBB2 (11.6%), PIK3CA (11.6%), STK11 (11.6%), IDH1/2 (7.8%), NF1/2 (7.8%), MTAP (7.0%).
Key Findings
- First real-world comparison of GCD (chemoimmunotherapy) and GCS (triplet chemotherapy with S-1) in advanced BTC.
- OS and PFS were comparable between regimens, with median OS 17.1 mounths in the GCD group versus 18.3 months months and median PFS was 8.0 months in the GCD group versus 8.9 months) in the GCS group.
- GCS produced a significantly longer duration of response (12.7 vs 7.5 months) and a higher proportion of durable responders at 9 and 12 months.
- Toxicity profiles were manageable for both regimens; grade ≥3 AEs were more common with GCD, but primarily hematologic.
- Exploratory genomic analyses suggested that patients with ARID1A or PIK3CA alterations or SMAD4 wild-type tumors may derive greater OS benefit from GCS than from GCD.
- In the Japanese setting, GCS is substantially less costly per treatment cycle than GCD and requires fewer hospital visits per month.
Conclusion
This single-center retrospective study shows that durvalumab plus gemcitabine/cisplatin (GCD) and gemcitabine/cisplatin/S-1 (GCS) provide comparable survival and overall disease control in patients with advanced or recurrent biliary tract cancer treated in routine practice. While GCD confirms the benefit of chemoimmunotherapy observed in phase III trials, GCS achieves similar OS with a significantly longer duration of response and a favorable cost and scheduling profile in the Japanese healthcare setting.
The exploratory genomic findings suggest that certain alterations—particularly in ARID1A, PIK3CA, and SMAD4—may influence the relative benefit of these regimens, although this requires confirmation in larger, prospective cohorts. Overall, the study supports positioning GCS alongside GCD as a standard first-line option for advanced BTC and underscores the need for biomarker-driven treatment selection and randomized trials directly comparing GCS and chemoimmunotherapy-based strategies.
You can read the full article here.


