Original Study Title: Durvalumab after radiotherapy in patients with unresectable stage III non-small-cell lung cancer ineligible for chemotherapy: the DUART phase II nonrandomized controlled study
Patients with unresectable stage III non small cell lung cancer (NSCLC) who are ineligible for chemotherapy typically receive radiotherapy (RT) alone, but outcomes are poor (historically ~8–11 months median OS). The PACIFIC regimen established durvalumab after concurrent chemoradiotherapy (cCRT) as standard for fit patients, but left a gap for frailer, older individuals who cannot receive chemotherapy.
DUART (NCT04249362) asked whether durvalumab after RT alone—either definitive or palliative dose—could be safe, tolerable, and clinically active in this population. The study also explored circulating tumor DNA (ctDNA) as a prognostic biomarker.
Methods and Study Design
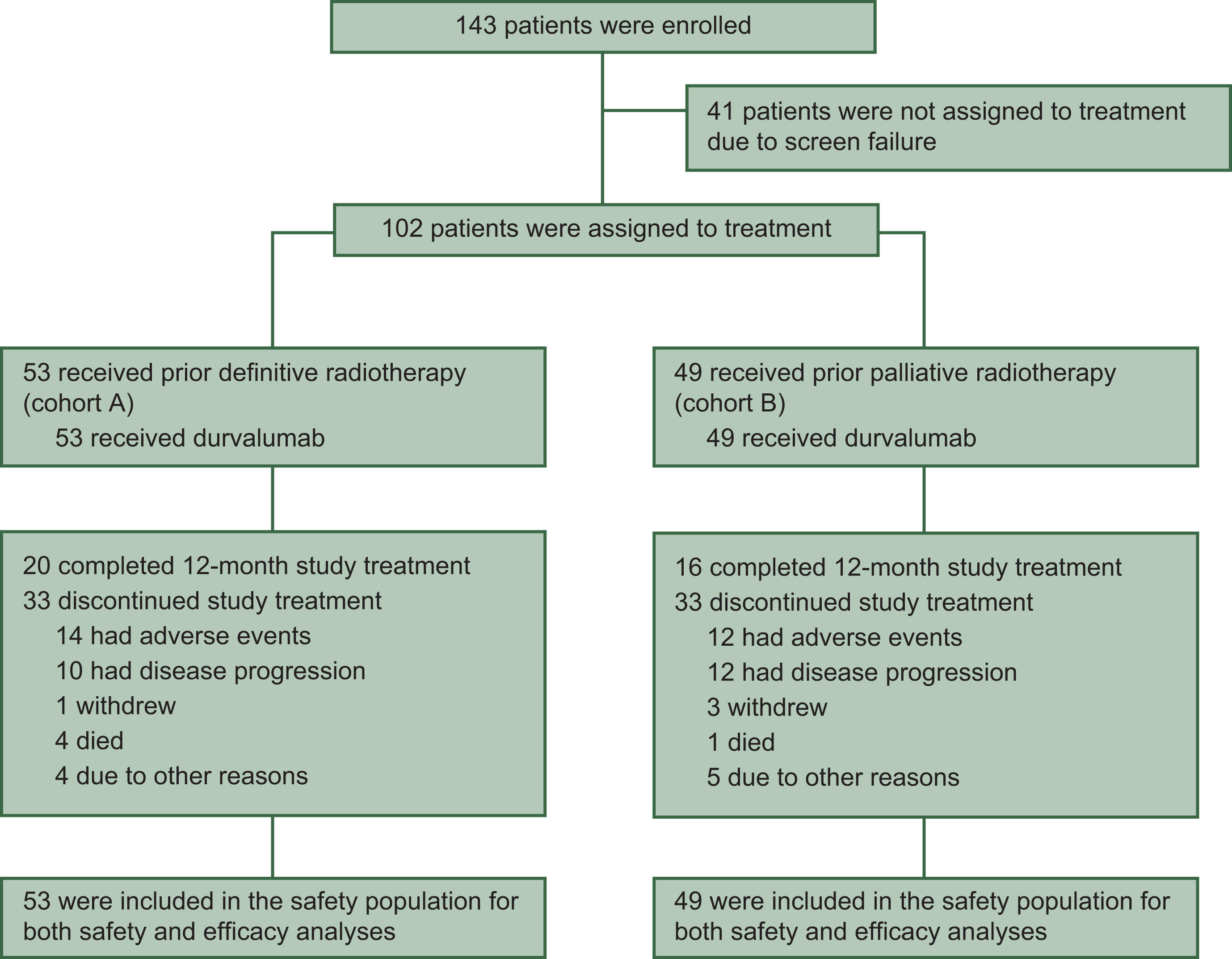
DUART was a phase II, open-label, single-arm, multicenter, international study that enrolled chemotherapy-ineligible patients with unresectable stage III NSCLC. The trial was nonrandomized but included two parallel cohorts defined by the radiotherapy (RT) dose patients had already received: a definitive-RT cohort (cohort A) and a palliative-RT cohort (cohort B).
In total, 102 patients initiated study treatment; they were elderly and frail by design (median age 79 years, 65% ≥75 years) with ECOG performance status 0, 1, or 2 in 19%, 73%, and 8% of patients, respectively. Cohort A (n=53) had completed definitive RT at 60 Gy ±10% (or biologically equivalent dose), whereas cohort B (n=49) had completed palliative RT at 40 to <54 Gy (or equivalent). All participants then received durvalumab 1500 mg intravenously every 4 weeks for up to 12 months; investigators could continue durvalumab beyond progression or beyond one year if they judged ongoing clinical benefit.
The primary endpoint was safety—specifically, the incidence of grade 3/4 possibly related adverse events (PRAEs) occurring within the first six months after starting durvalumab. Secondary endpoints assessed antitumor activity and broader safety, including objective response rate (ORR), progression-free survival (PFS), overall survival (OS), duration of response (DoR), lung cancer–specific survival, and summaries of adverse events (AEs), serious AEs (SAEs), adverse events of special interest (AESIs), and immune-mediated AEs.
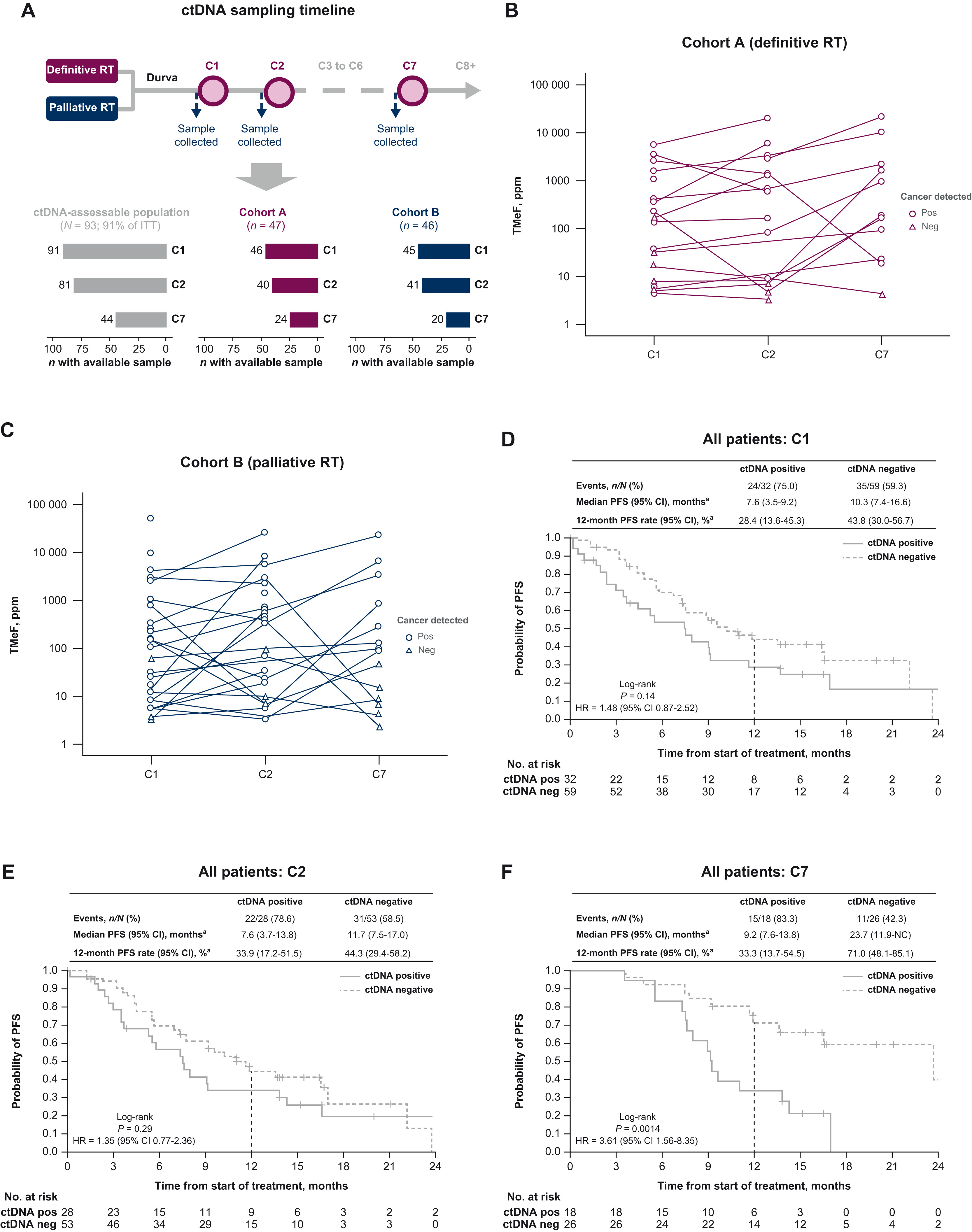
Exploratory analyses investigated biomarkers: tumor PD-L1 expression using the VENTANA SP263 assay with a 1% tumor-cell cutoff, and circulating tumor DNA (ctDNA) dynamics measured by a targeted methylation assay at pre-dose time points for cycles 1, 2, and 7.
Results of the Study
Between November 2020 and September 2022, 143 patients were screened and 102 were treated across 29 centers in Europe (Italy, Poland, France, Spain, and Russia). The median age was 79 years, with nearly two-thirds (65%) aged ≥75 years. Most patients were male (72%) and had ECOG performance status 1 (73%). Stage IIIA disease was most common (61%), followed by IIIB (32%) and IIIC (6%). Histology was squamous in 56%, adenocarcinoma in 34%, and other types in 10%.
Safety outcomes
Nearly all patients (97%) experienced at least one adverse event (AE) of any grade, with 40% reporting grade 3/4 AEs. The most frequent AEs included cough, asthenia, anemia, dyspnea, and pyrexia. Within 6 months of durvalumab initiation, 9.8% (10 patients) developed grade 3/4 possibly related adverse events (PRAEs), meeting the primary endpoint. The most common grade 3/4 PRAEs were asthenia, hepatotoxicity, and pneumonia (each 2%). Pneumonitis was the most frequent reason for durvalumab discontinuation, occurring in 4.9% of patients; one fatal case of pneumonitis was reported. Overall, 21.6% of patients discontinued durvalumab due to AEs, and 47% required at least one treatment interruption.
Efficacy outcomes
Durvalumab demonstrated encouraging clinical activity in this frail, chemotherapy-ineligible population. Median progression-free survival (PFS) was 9.2 months (95% CI 7.4–11.9), with a 12-month PFS rate of 39.6%. Median overall survival (OS) was 21.1 months (95% CI 14.8–not calculable), and the 12-month OS rate was 64.7%. Outcomes were generally more favorable in patients who had received definitive RT (cohort A) compared with palliative RT (cohort B). Median PFS was 10.3 months in cohort A versus 7.6 months in cohort B, with 12-month PFS rates of 46.8% and 31.8%, respectively. Median OS was 21.1 months in cohort A and 16.8 months in cohort B.
Response outcomes
The confirmed objective response rate (ORR) was 34.0% (95% CI 21.5–48.3) in cohort A and 24.5% (95% CI 13.3–38.9) in cohort B. Two complete responses were observed, both in the definitive RT cohort. Median duration of response was 56.9 weeks in cohort A and 34.1 weeks in cohort B.
Biomarker analyses
PD-L1 status was evaluable in 71 patients; outcomes tended to be better in those with tumor-cell PD-L1 expression ≥1% compared with <1%. In exploratory ctDNA analyses, patients who remained ctDNA-positive during durvalumab treatment—particularly at cycle 7—had significantly shorter PFS compared with ctDNA-negative patients, suggesting that ctDNA may be a useful biomarker of prognosis and treatment response.
Key Takeaway Messages
- Feasible and tolerable: Durvalumab after RT alone (without chemotherapy) shows a manageable safety profilein a frail, elderly stage III NSCLC population, with <10% grade 3/4 PRAEs in the first 6 months.
- Clinically encouraging activity: Median OS ~21 months and PFS ~9 months compare favorably to historical RT-alone outcomes in CT-ineligible patients. Outcomes were better after definitive RT, as expected, but palliative RT + durvalumab still yielded meaningful disease control.
- Biomarker signal: On-treatment ctDNA negativity correlates with longer PFS and may identify patients achieving molecular remission; ctDNA positivity flags higher-risk patients.
- Practice implications: For patients ineligible for chemotherapy, durvalumab consolidation after RT—including after palliative RT—is a reasonable option that merits consideration where PACIFIC is not feasible.
- Next steps: Randomized comparisons vs RT alone, prospective ctDNA-guided strategies, and refinement of PD-L1/methylation-based biomarkers to personalize post-RT immunotherapy in this population.
You can read all article here.


